Advertisements
Advertisements
Question
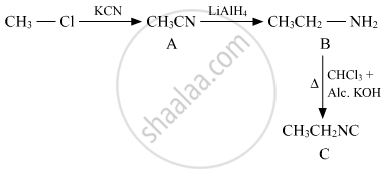
Write the structures of A, B and C in the following:

Solution
APPEARS IN
RELATED QUESTIONS
Given reasons: C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
Which compound in the following pair reacts faster in SN2 reaction with OH–?
- CH3Br or CH3
- CH3Cl, (CH3)3CCl
An important chemical method to resolve a racemic mixture makes use of the formation of ______.
Racemic compound has ____________.
Read the passage given below and answer the following question:
Nucleophilic substitution reaction of haloalkane can be conducted according to both SN1 and SN2 mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent.
Influences of halogen: No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks the central carbon atom with electron pair. Therefore, the weaker the alkalinity of leaving group is, the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom; that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I− < Br− < Cl− < F− and the order of their leaving tendency should be I− > Br− > Cl− > F−. Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI > RBr > RCl > RF. In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on SN1 mechanism. If the leaving group is not easy to leave, the reaction is based on SN2 a mechanism.
Influences of solvent polarity: In SN1 reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In SN2 reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate. For example, the decomposition rate (SN1) of tertiary chlorobutane in 25℃ water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both SN1 and SN2 reactions, but with different results. Generally speaking, weak polar solvent is favorable for SN2 reaction, while strong polar solvent is favorable for SN1 reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example, ethanol containing water).
Nucleophilic substitution will be fastest in case of ______.
Compound ‘A’ with molecular formula \[\ce{C4H9Br}\] is treated with aq. \[\ce{KOH}\] solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. \[\ce{KOH}\] solution, the rate of reaction was found to be dependent on concentration of compound and \[\ce{KOH}\] both.
(i) Write down the structural formula of both compounds ‘A’ and ‘B’.
(ii) Out of these two compounds, which one will be converted to the product with inverted configuration.
Give reason for the following:
The product formed during SN1 reaction is a racemic mixture.
Assertion (A) : Nucleophilic substitution of iodoethane is easier than chloroethane.
Reason (R) : Bond enthalpy of C-I bond is less than that of C-Cl bond.
Explain why Grignard reagents should be prepared under anhydrous conditions.
