Advertisements
Advertisements
प्रश्न
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
उत्तर
The SN2 reaction involves the formation of a transition state with the carbon atom surrounded by 5 additional atoms (groups). A transition state requires minimum steric interactions. The most suitable substrates for SN2 reactions are 1° alkyl halides, followed by 2° and 3° alkyl halides. The order of reactivity towards SN2 is 1° > 2° > 3°> aryl halide. Based on this, the order will be
\[\begin{array}{cc}
\ce{CH3}\phantom{...............................}\ce{CH3}\phantom{.................}\ce{CH3}\phantom{.....}\\
\phantom{.....}|\phantom{...................................}|\phantom{....................}|\phantom{............}\\
\ce{\underset{1-Bromo-3-methylbutane}{H3C - CH - CH2 - CH2Br} > \ce{CH3 - CH - CH - CH3} > \ce{CH3 - C - CH2 - CH3}}\\
\phantom{.......................}|\phantom{..........................}|\\
\phantom{.........................}\ce{\underset{2-Bromo-3-methylbutane}{Br}\phantom{.........}\ce{\underset{2-Bromo-2-methylbutane}{Br}}}\
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
How will you bring about the following conversion?
Toluene to benzyl alcohol
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
What happens when ethyl chloride is treated with aqueous KOH?
C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
The stability order for carbocation is _______.
(A) 2° > 3° > 1°
(B) 3° > 2° > 1°
(C) 3° > 1° > 2°
(D) 1° > 3° > 2°
Given reasons: SN1 reactions are accompanied by racemization in optically active alkyl halides.
What is the action of the following on ethyl bromide
alcoholic solution of potassium hydroxide.
Identify X and Y in the following sequence:
\[\ce{C2H5Br ->[X] Product ->[Y] C3H7NH2}\]
Which of the following statements are correct about the kinetics of this reaction?
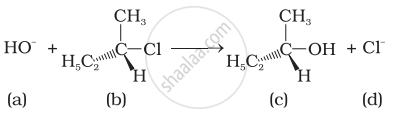
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
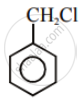
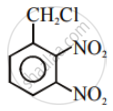
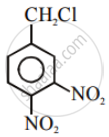
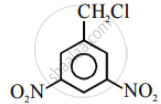
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 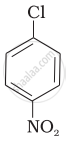 |
| (II) | 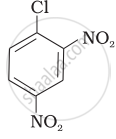 |
| (III) | 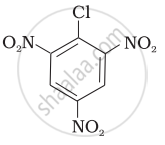 |
The major product formed in the following reaction is:
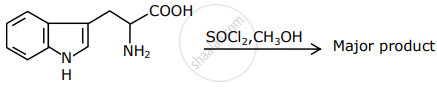
In SN1 reactions, the correct order of reactivity for the following compounds:
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is ______.
Optical activity of an enantiomeric mixture is +12.6° and the specific rotation of (+) isomer is +30°. The optical purity is ______ %.
The decreasing order of reactivity of the following compounds towards nucleophilic substitution (SN2) is ______.
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
|
Nucleophilic Substitution: Influences of solvent polarity: The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. Hence the level of solvent polarity has an influence on both SN1 and SN2 reactions but with different results. Generally speaking, a weak polar solvent is favourable for SN2 reaction, while a strong polar solvent is favourable for SN1. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example ethanol containing water). |
Answer the following questions:
(a) Why racemisation occurs in SN1? (1)
(b) Why is ethanol less polar than water? (1)
(c) Which one of, the following in each pair is more reactive towards SN2 reaction? (2)
(i) CH3 – CH2 – I or CH3CH2 – Cl
(ii)
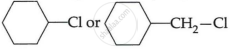
OR
(c) Arrange the following in the increasing order of their reactivity towards SN1 reactions: (2)
(i) 2-Bromo-2-methylbutane, 1-Bromo-pentane, 2-Bromo-pentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3- methylbutane
Explain why Grignard reagents should be prepared under anhydrous conditions.
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CH2CHCH3}\\\phantom{...}|\\\phantom{....}\ce{Br}\end{array}\] or \[\begin{array}{cc}\phantom{.....}\ce{CH3}\\\phantom{..}|\\\ce{H3C - C - Br}\\\phantom{..}|\\\phantom{....}\ce{CH3}\end{array}\]