Advertisements
Advertisements
Question
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Solution
SN2 reaction involves the formation of transition state. Therefore, reactivity towards SN2 depends upon the steric hindrance:
Due to the presence of 2 methyl group in 2-Bromo-2-methylbutane it is least reactive towards SN2 reactions, 2-bromopentane is moderately reactive while 1-bromopentane is most reactive.
2-Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
APPEARS IN
RELATED QUESTIONS
Which would undergo SN2 reaction faster in the following pair and why ?
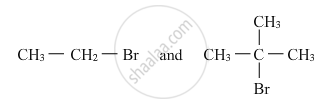
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
CH3CH2CH2CH2Br or \[\begin{array}{cc}
\ce{CH3CH2CHCH3}\\
\phantom{...}|\\
\phantom{....}\ce{Br}\
\end{array}\]
Write the isomers of the compound having the formula C4H9Br.
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
Halogenation of alkanes is ____________.
Optically active isomers but not mirror images are called ____________.
Which of the following is an optically active compound?
Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
The number of chiral alcohol (s) with molecular formula C4H10O is ______.
Complete the reaction with the main product formed:

