Advertisements
Advertisements
प्रश्न
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
उत्तर
The SN2 reaction involves the formation of a transition state with the carbon atom surrounded by 5 additional atoms (groups). A transition state requires minimum steric interactions. The most suitable substrates for SN2 reactions are 1° alkyl halides, followed by 2° and 3° alkyl halides. The order of reactivity towards SN2 is 1° > 2° > 3°> aryl halide. Based on this, the order will be
\[\begin{array}{cc}
\phantom{.....................}\ce{Br}\phantom{...........................}\ce{Br}\\
\phantom{...................}|\phantom{.............................}|\\
\ce{\underset{1-Bromopentane}{H3C - (CH2)3 - CH2Br} > \ce{\underset{2-Bromopentane}{CH3 - CH - (CH2)2 - CH3}} > \ce{CH3 - C - CH2 - CH3}}\\
\phantom{.................................................}|\\
\phantom{....................................................}\ce{\underset{2-Brmo-2-methylbutane}{CH3}}\
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
Write the isomers of the compound having the formula C4H9Br.
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
What is the action of the following on ethyl bromide
alcoholic solution of potassium hydroxide.
What is the action of the following on ethyl bromide?
moist silver oxide
AgCN reacts with haloalkanes to form isocyanide. Haloalkanes react with KCN to form alkyl cyanides as the main product. Why?
Arrange the following organic compounds in descending order of their reactivity towards SN1 reaction.
C6H5CH2Br, C6H5CH(C6H5)Br, C6H5CH(CH3)Br, C6H5C(CH3)(C6H5)Br
Which one of the following halogen compounds is difficult to be hydrolysed by SN1 mechanism?
Most reactive halide towards SN1 reaction is ____________.
Optically active isomers but not mirror images are called ____________.
SN2 mechanism proceeds through intervention of ____________.
Tertiary alkyl halides are practically inert to substitution by SN2 mechanism because of ____________.
The reaction of C6H5–CH=CH–CH3 with HBr produces:
Which of the following statements are correct about the kinetics of this reaction?
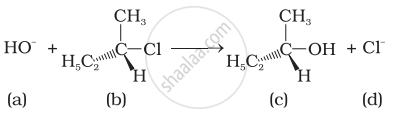
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Chlorination of alkanes is an example of
CCl4 is insoluble in water because:-
In SN1 reactions, the correct order of reactivity for the following compounds:
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is ______.
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
Discuss SN2 mechanism of methyl bromide using aqueous KOH.
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CHCH2CH2Br}\\|\phantom{.........}\\\ce{CH3}\phantom{......}\end{array}\] or \[\begin{array}{cc}\ce{CH3CH2CHCH2Br}\\\phantom{}|\\\phantom{...}\ce{CH3}\end{array}\]
