Advertisements
Advertisements
Question
Given reasons: SN1 reactions are accompanied by racemization in optically active alkyl halides.
Solution
In SN1 reaction, formation of carbocation as an intermediate takes place. This carbocation has sp2-hybridised and planar structure. This planar carbocation is attacked by nucleophile from both the sides equally to form d and l isomers in equal proportion. Such products are called racemic mixture. Hence, SN1 reactions are accompanied by racemisation in optically active alkyl halides.
RELATED QUESTIONS
What happens when chlorobenzene is subjected to hydrolysis?
Which of the following is optically inactive?
Which one is most reactive towards SN1 reaction?
Read the passage given below and answer the following question:
Nucleophilic substitution reaction of haloalkane can be conducted according to both SN1 and SN2 mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent.
Influences of halogen: No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks the central carbon atom with electron pair. Therefore, the weaker the alkalinity of leaving group is, the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom; that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I− < Br− < Cl− < F− and the order of their leaving tendency should be I− > Br− > Cl− > F−. Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI > RBr > RCl > RF. In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on SN1 mechanism. If the leaving group is not easy to leave, the reaction is based on SN2 a mechanism.
Influences of solvent polarity: In SN1 reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In SN2 reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate. For example, the decomposition rate (SN1) of tertiary chlorobutane in 25℃ water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both SN1 and SN2 reactions, but with different results. Generally speaking, weak polar solvent is favorable for SN2 reaction, while strong polar solvent is favorable for SN1 reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example, ethanol containing water).
SN1 reaction will be fastest in which of the following solvents?
Which of the following statements are correct about this reaction?
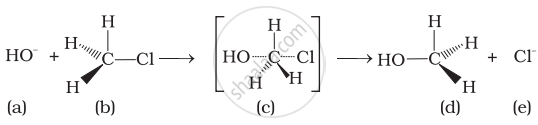
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Which of the following is the definition of chirality?
Which one is the correct order of nucleophilic strength (pKa) of following nucleophiles?
The number of chiral alcohol (s) with molecular formula C4H10O is ______.
Discuss SN2 mechanism of methyl bromide using aqueous KOH.
