Advertisements
Advertisements
Question
What happens when chlorobenzene is subjected to hydrolysis?
Solution
Chlorobenzene does not undergo hydrolysis under normal conditions. However, it undergoes hydrolysis when heated in an aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atm to form phenol.

APPEARS IN
RELATED QUESTIONS
Which would undergo SN1 reaction faster in the following pair and why?
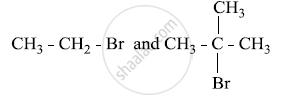
In the following pair of halogen compounds, which compound undergoes a faster SN1 reaction?
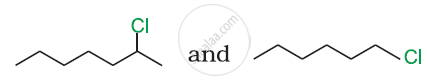
Which compound in the following pair will react faster in SN2 reaction with OH−?
(CH3)3CCl or CH3Cl
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
What is the action of the following on ethyl bromide
alcoholic solution of potassium hydroxide.
Answer the following question.
Write one stereochemical difference between SN1 and SN2 reactions.
Which of the following is an example of SN2 reaction?
Most reactive halide towards SN1 reaction is ____________.
Tertiary alkyl halides are practically inert to substitution by SN2 mechanism because of ____________.
2-Bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is ____________.
Which of the following statements are correct about this reaction?
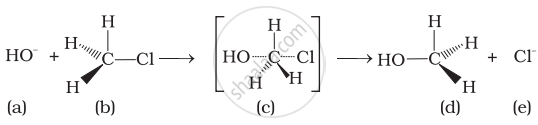
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
How do polar solvents help in the first step in SN1 mechanism?
Match the reactions given in Column I with the types of reactions given in Column II.
| Column I | Column II | |
| (i) |  |
(a) Nucleophilic aromatic substitution |
| (ii) | \[\begin{array}{cc} \ce{CH3 - CH = CH2 + HBr -> CH3 - CH - CH3}\\ \phantom{............................}|\phantom{}\\ \phantom{.............................}\ce{Br}\phantom{} \end{array}\] |
(b) Electrophilic aromatic substitution |
| (iii) | 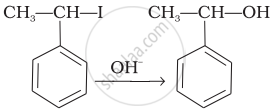 |
(c) Saytzeff elimination |
| (iv) | 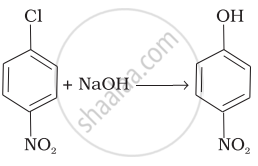 |
(d) Electrophilic addition |
| (v) | \[\begin{array}{cc} \ce{CH3 CH2 CH CH3 ->[alc.KOH] CH3 CH = CH CH3}\\ \phantom{}|\phantom{..........................}\\ \phantom{}\ce{Br}\phantom{........................} \end{array}\] |
(e) Nucleophilic substitution (SN1) |
In SN1 reactions, the correct order of reactivity for the following compounds:
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is ______.
Complete the reaction with the main product formed:

The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
|
Nucleophilic Substitution: Influences of solvent polarity: The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. Hence the level of solvent polarity has an influence on both SN1 and SN2 reactions but with different results. Generally speaking, a weak polar solvent is favourable for SN2 reaction, while a strong polar solvent is favourable for SN1. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example ethanol containing water). |
Answer the following questions:
(a) Why racemisation occurs in SN1? (1)
(b) Why is ethanol less polar than water? (1)
(c) Which one of, the following in each pair is more reactive towards SN2 reaction? (2)
(i) CH3 – CH2 – I or CH3CH2 – Cl
(ii)
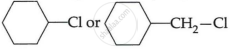
OR
(c) Arrange the following in the increasing order of their reactivity towards SN1 reactions: (2)
(i) 2-Bromo-2-methylbutane, 1-Bromo-pentane, 2-Bromo-pentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3- methylbutane
Acetic anhydride from acetic acid
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CH2CHCH3}\\\phantom{...}|\\\phantom{....}\ce{Br}\end{array}\] or \[\begin{array}{cc}\phantom{.....}\ce{CH3}\\\phantom{..}|\\\ce{H3C - C - Br}\\\phantom{..}|\\\phantom{....}\ce{CH3}\end{array}\]
