Advertisements
Advertisements
प्रश्न
What are ambident nucleophiles? Explain with an example.
उत्तर
Ambident nucleophiles attack alkyl halides through two distinct atoms. This occurs due to the presence of two nucleophilic centres resulting from resonance structures in the ion. For example, the lone pair of electrons on N in the NO2 ion makes it nucleophilic, while oxygen's negative charge works as a nucleophile. Thus, NO2 can attack the O or N atoms, making it ambidentate.
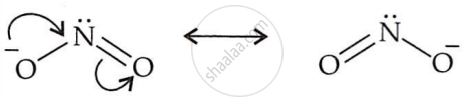
\[\ce{RX + Ag - O - N = O -> \underset{Nitroalkane}{R - NO2}}\]
\[\ce{RX + KNO2 -> \underset{Alkyl nitrate}{R - O - N = O}}\]
संबंधित प्रश्न
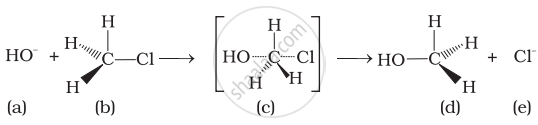
Discuss the mechanism of alkaline hydrolysis of bromomethane.
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2Br + KCN ->[aq.ethanol]}\]
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
Given reasons: The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
In the reaction, \[\ce{R - X + NaOR' -> ROR’ + X}\] ( – ve ion). The main product formed is:
Which of the following is an example of SN2 reaction?
Racemic compound has ____________.
Which of the following is a chiral compound?
The increasing order of reactivity towards SN1 mechanism is:
(I) \[\begin{array}{cc}
\ce{CH3-CH-CH2-CH3}\\
|\phantom{........}\\
\ce{CH3}\phantom{.....}
\end{array}\]
(II) CH3CH2CH2Cl
(III) P–CH3O–C6H4–CH2Cl
Which of the following statements are correct about this reaction?
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
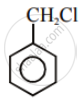
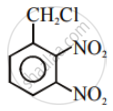
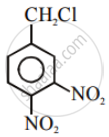
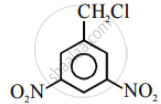
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 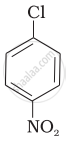 |
| (II) | 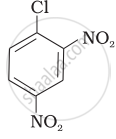 |
| (III) | 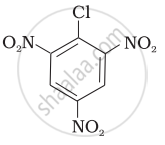 |
Chlorination of alkanes is an example of
Which of the following is the definition of chirality?
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
The decreasing order of reactivity of the following compounds towards nucleophilic substitution (SN2) is ______.
Arrange the following compounds in increasing order of reactivity towards SN2 reaction.
2-Bromopentane, 1-Bromopentane, 2-Bromo-2-methylbutane
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
|
Nucleophilic Substitution: Influences of solvent polarity: The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. Hence the level of solvent polarity has an influence on both SN1 and SN2 reactions but with different results. Generally speaking, a weak polar solvent is favourable for SN2 reaction, while a strong polar solvent is favourable for SN1. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example ethanol containing water). |
Answer the following questions:
(a) Why racemisation occurs in SN1? (1)
(b) Why is ethanol less polar than water? (1)
(c) Which one of, the following in each pair is more reactive towards SN2 reaction? (2)
(i) CH3 – CH2 – I or CH3CH2 – Cl
(ii)
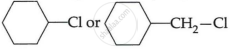
OR
(c) Arrange the following in the increasing order of their reactivity towards SN1 reactions: (2)
(i) 2-Bromo-2-methylbutane, 1-Bromo-pentane, 2-Bromo-pentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3- methylbutane
Identify the product in the following reaction: 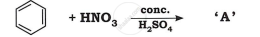
Discuss SN2 mechanism of methyl bromide using aqueous KOH.