Advertisements
Advertisements
प्रश्न
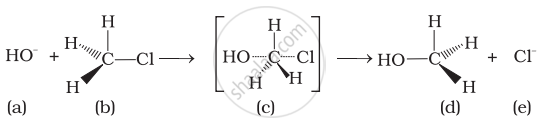
Which of the statements are correct about above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridised.
(iii) In (c) carbon atom is sp2 hybridised.
(iv) (a) and (e) both are electrophiles.
उत्तर
(i) (a) and (e) both are nucleophiles.
(iii) In (c) carbon atom is sp2 hybridised.
Explanation:
HO− and Cl− are nucleophiles.
In (iii), C atom is sp2 hybridised due to formation of \[\ce{C - OH}\] bond and breaking of \[\ce{C - Cl}\] bond simultaneously. So, in the transition state, the C atom is bonded to only 3 H atoms completely.
APPEARS IN
संबंधित प्रश्न
Which would undergo SN1 reaction faster in the following pair and why?
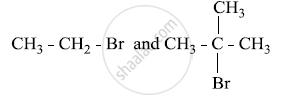
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2CH2OH + SOCl2 ->}\]
Which of the following is an example of SN2 reaction?
Which of the following pairs is/are correctly matched?
| Reaction | Product | |
| I | RX + AgCN | RNC |
| II | RX + KCN | RCN |
| III | RX + KNO2 | \[\begin{array}{cc} \phantom{.......}\ce{O}\\ \phantom{.....}/\\ \ce{R - N}\phantom{....}\\ \phantom{.....}\backslash\backslash\\ \phantom{.......}\ce{O} \end{array}\] |
| IV | RX + AgNO2 | \[\ce{R-O-N=O}\] |
Most reactive halide towards SN1 reaction is ____________.
2-Bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is ____________.
SN1 reaction of alkyl halides lead to ___________.
Which of the following alkyl halides will undergo SN1 reaction most readily?
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 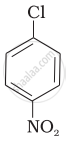 |
| (II) | 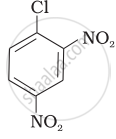 |
| (III) | 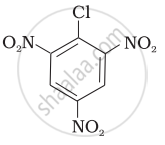 |
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
|
Nucleophilic Substitution: Influences of solvent polarity: The reaction rate (SN2) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. Hence the level of solvent polarity has an influence on both SN1 and SN2 reactions but with different results. Generally speaking, a weak polar solvent is favourable for SN2 reaction, while a strong polar solvent is favourable for SN1. Generally speaking, the substitution reaction of tertiary haloalkane is based on SN1 mechanism in solvents with a strong polarity (for example ethanol containing water). |
Answer the following questions:
(a) Why racemisation occurs in SN1? (1)
(b) Why is ethanol less polar than water? (1)
(c) Which one of, the following in each pair is more reactive towards SN2 reaction? (2)
(i) CH3 – CH2 – I or CH3CH2 – Cl
(ii)
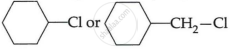
OR
(c) Arrange the following in the increasing order of their reactivity towards SN1 reactions: (2)
(i) 2-Bromo-2-methylbutane, 1-Bromo-pentane, 2-Bromo-pentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3- methylbutane
