Advertisements
Online Mock Tests
Chapters
2: Solutions
3: Electrochemistry
4: Chemical Kinetics
5: Surface Chemistry
6: General Principle and Processes of Isolation of Elements
7: The p-block Elements
8: The d-and f-Block Elements
9: Coordination Compounds
▶ 10: Haloalkanes and Haloarenes
11: Alcohols, Phenols and Ethers
12: Aldehydes, Ketones and Carboxylic Acids
13: Amines
14: Biomolecules
15: Polymers
16: Chemistry In Everyday Life
![NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 10 - Haloalkanes and Haloarenes NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 10 - Haloalkanes and Haloarenes - Shaalaa.com](/images/chemistry-english-class-12_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 10: Haloalkanes and Haloarenes
Below listed, you can find solutions for Chapter 10 of CBSE NCERT Exemplar for Chemistry [English] Class 12.
NCERT Exemplar solutions for Chemistry [English] Class 12 10 Haloalkanes and Haloarenes Exercises [Pages 133 - 149]
Multiple Choice Questions (Type-I)
The order of reactivity of alcohols with halogen acids is ______.
(A) \[\ce{CH3CH2 - CH2 - OH}\]
(B) \[\begin{array}{cc}
\phantom{}\ce{CH3CH2 - CH - OH}\\
\phantom{...}\phantom{}|\\
\phantom{......}\ce{CH3}
\end{array}\]
(C) \[\begin{array}{cc}
\phantom{........}\ce{CH3}\\
\phantom{.....}\phantom{}|\\
\phantom{}\ce{CH3CH2 - C - OH}\\
\phantom{.....}\phantom{}|\\
\phantom{........}\ce{CH3}
\end{array}\]
(A) > (B) > (C)
(C) > (B) > (A)
(B) > (A) > (C)
(A) > (C) > (B)
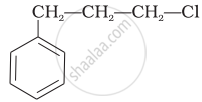
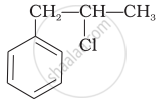
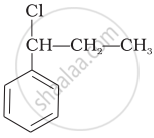
Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
\[\ce{CH3CH2 - CH2 - OH}\]
\[\begin{array}{cc}
\phantom{}\ce{CH3CH2 - CH - OH}\\ \phantom{...}\phantom{}|\\
\phantom{......}\ce{CH3}
\end{array}\]\[\begin{array}{cc}
\ce{CH3CH2-CH-CH2OH}\\
|\phantom{..}\\
\phantom{.}\ce{CH3}
\end{array}\]\[\begin{array}{cc}
\phantom{........}\ce{CH3}\\
\phantom{.....}\phantom{}|\\
\phantom{}\ce{CH3CH2 - C - OH}\\
\phantom{.....}\phantom{}|\\ \phantom{........}\ce{CH3}
\end{array}\]
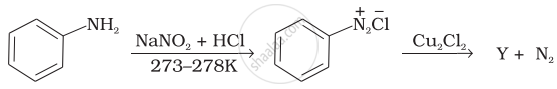
Identify the compound Y in the following reaction.
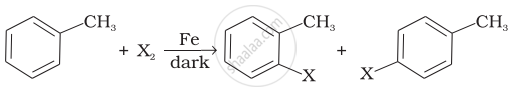
Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is ______.
Electrophilic elimination reaction
Electrophilic substitution reaction
Free radical addition reaction
Nucleophilic substitution reaction
Which of the following is halogen exchange reaction?
\[\ce{RX + NaI -> RI + NaX}\]
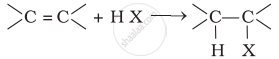
\[\ce{R-OH + HX ->[ZnCl2] R-X + H2O}\]
Which reagent will you use for the following reaction?
\[\ce{CH3CH2CH2CH3 -> CH3CH2CH2CH2Cl + CH3CH2CHClCH3}\]
Cl2/UV light
\[\ce{NaCl + H2CO4}\]
Cl2 gas in dark
Cl2 gas in the presence of iron in dark
Arrange the following compounds in the increasing order of their densities.
(a)
(b)
(c)
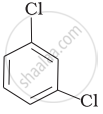
(d)
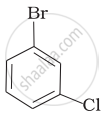
(a) < (b) < (c) < (d)
(a) < (c) < (d) < (b)
(d) < (c) < (b) < (a)
(b) < (d) < (c) < (a)
Arrange the following compounds in increasing order of their boiling points.
(a) \[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
\backslash\phantom{.............}\\
\ce{CH - CH2Br}\\
/\phantom{.............}\\
\ce{CH3}\phantom{.................}
\end{array}\]
(b) \[\ce{CH3CH2CH2CH2Br}\]
(c) \[\begin{array}{cc}
\phantom{...}\ce{CH3}\\
\phantom{}|\\
\ce{H3C - C - CH3}\\
\phantom{}|\\
\phantom{..}\ce{Br}
\end{array}\]
(b) < (a) < (c)
(a) < (b) < (c)
(c) < (a) < (b)
(c) < (b) < (a)
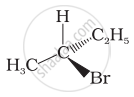
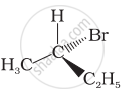
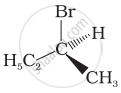
In which of the following molecules carbon atom marked with asterisk (*) is asymmetric?
(a) \[\begin{array}{cc}
\ce{H}\\
|\\
\phantom{.}\ce{C^*}\phantom{}\\
\phantom{}/\phantom{..}|\phantom{..}\backslash\phantom{}\\
\phantom{.}\ce{I}\phantom{...}\ce{Br}\phantom{..}\ce{Cl}
\end{array}\]
(b) \[\begin{array}{cc}
\ce{D}\\
|\\
\phantom{.}\ce{C^*}\phantom{}\\
\phantom{}/\phantom{..}|\phantom{..}\backslash\phantom{}\\
\phantom{.}\ce{I}\phantom{...}\ce{Br}\phantom{..}\ce{Cl}
\end{array}\]
(c) \[\begin{array}{cc}
\ce{H}\\
|\\
\phantom{.}\ce{C^*}\phantom{}\\
\phantom{}/\phantom{..}|\phantom{..}\backslash\phantom{}\\
\phantom{..}\ce{OH}\phantom{..}\ce{C2H5}\phantom{}\ce{CH3}
\end{array}\]
(d) \[\begin{array}{cc}
\ce{H}\\
|\\
\phantom{.}\ce{C^*}\phantom{}\\
\phantom{}/\phantom{..}|\phantom{..}\backslash\phantom{}\\
\phantom{..}\ce{H}\phantom{...}\ce{C2H5}\phantom{}\ce{CH3}
\end{array}\]
(a), (b), (c), (d)
(a), (b), (c)
(b), (c), (d)
(a), (c), (d)
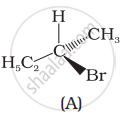
Which of the following structures is enantiomeric with the molecule (A) given below:
Which of the following is an example of vic-dihalide?
Dichloromethane
1, 2-dichloroethane
Ethylidene chloride
Allyl chloride
The position of \[\ce{-Br}\] in the compound in \[\ce{CH3CH = CH(Br)(CH3)2}\] can be classified as ______.
Allyl
Aryl
Vinyl
Secondary
Chlorobenzene is formed by reaction of chlorine with benzene in the presence of \[\ce{AlCl3}\]. Which of the following species attacks the benzene ring in this reaction?
\[\ce{Cl-}\]
\[\ce{Cl+}\]
\[\ce{AlCl3}\]
\[\ce{[AlCl4]-}\]
Ethylidene chloride is a/an ______.
vic-dihalide
gem-dihalide
allylic halide
vinylic halide
What is ‘A’ in the following reaction?
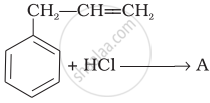
A primary alkyl halide would prefer to undergo ______.
SN1 reaction
SN2 reaction
α–Elimination
Racemisation
Which of the following alkyl halides will undergo SN1 reaction most readily?
\[\ce{(CH3)3C-F}\]
\[\ce{(CH3)3C-Cl}\]
\[\ce{(CH3)3C-Br}\]
\[\ce{(CH3)3C-I}\]
Which is the correct IUPAC name for \[\begin{array}{cc}
\ce{CH3 - CH - CH2 - Br}\\
\phantom{}|\phantom{.......}\\
\phantom{}\ce{C2H5}\phantom{....}
\end{array}\]?
1-Bromo-2-ethylpropane
1-Bromo-2-ethyl-2-methylethane
1-Bromo-2-methylbutane
2-Methyl-1-bromobutane
What should be the correct IUPAC name for diethylbromomethane?
1-Bromo-1,1-diethylmethane
3-Bromopentane
1-Bromo-1-ethylpropane
1-Bromopentane
The reaction of toluene with chlorine in the presence of iron and in the absence of light yields ______.
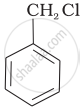
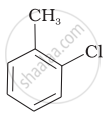
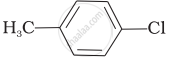
Mixture of
 and
and 
Chloromethane on treatment with excess of ammonia yields mainly ______.
N, N-Dimethylmethanamine
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\phantom{}\\
\phantom{.........}/{}\phantom{}\\
\phantom{}\ce{CH3 - N\phantom{..}\\
\phantom{.........}\backslash{}\phantom{}\\
\phantom{..............}\ce{CH3}\phantom{}\\
\ce{}}
\end{array}\]N–methylmethanamine (CH3—NH—CH3)
Methenamine (CH3NH2)
Mixture containing all these in equal proportion
Molecules whose mirror image is non-superimposable over them are known as chiral. Which of the following molecules is chiral in nature?
2-Bromobutane
1-Bromobutane
2-Bromopropane
2-Bromopropan-2-ol
Reaction of \[\ce{C6H5CH2Br}\] with aqueous sodium hydroxide follows ______.
SN1 mechanism
SN2 mechanism
Any of the above two depending upon the temperature of reaction
Saytzeff rule
Which of the carbon atoms present in the molecule given below are asymmetric?
\[\begin{array}{cc}
\phantom{}\ce{HO}\phantom{.....}\ce{OH}\phantom{..}\ce{H}\phantom{.....}\ce{O}\phantom{..}\\
\phantom{..}\backslash\phantom{.....}|\phantom{....}|\phantom{.....}//\phantom{.}\\
\ce{\overset{a}{C} - \overset{b}{C} - \overset{c}{C} - \overset{d}{C}}\\
\phantom{..}//\phantom{.....}|\phantom{....}|\phantom{....}\phantom{.}\backslash\phantom{...}\\
\phantom{}\ce{O}\phantom{......}\ce{H}\phantom{...}\ce{OH}\phantom{...}\ce{H}\phantom{}\\
\end{array}\]
a, b, c, d
b, c
a, d
a, b, c
Which of the following compounds will give racemic mixture on nucleophilic substitution by \[\ce{OH-}\] ion?
(a) \[\begin{array}{cc}
\phantom{}\ce{CH3 - CH - Br}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
(b) \[\begin{array}{cc}
\phantom{..}\ce{Br}\\
\phantom{}|\\
\phantom{}\ce{CH3 - C - CH3}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
(c) \[\begin{array}{cc}
\phantom{....}\ce{CH3 - CH - CH2Br}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
(a)
(a), (b), (c)
(b), (c)
(a), (c)
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 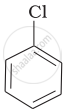 |
| (b) | 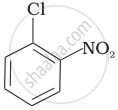 |
| (c) | 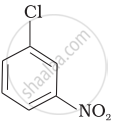 |
(a) < (b) < (c)
(c) < (b) < (a)
(a) < (c) < (b)
(c) < (a) < (b)
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 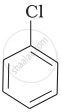 |
| (b) | 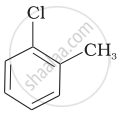 |
| (c) | 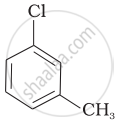 |
(a) < (b) < (c)
(a) < (c) < (b)
(c) < (b) < (a)
(b) < (c) < (a)
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 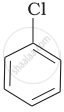 |
| (b) | 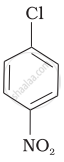 |
| (c) | 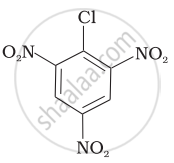 |
(c) < (b) < (a)
(b) < (c) < (a)
(a) < (c) < (b)
(a) < (b) < (c)
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 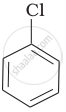 |
| (b) | 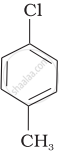 |
| (c) | 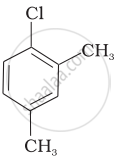 |
(a) < (b) < (c)
(b) < (a) < (c)
(c) < (b) < (a)
(a) < (c) < (b)
Which is the correct increasing order of boiling points of the following compounds?
1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane
Butane < 1-Chlorobutane < 1-Bromobutane < 1-Iodobutane
1-Iodobutane < 1-Bromobutane < 1-Chlorobutane < Butane
Butane < 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane
Butane < 1-Chlorobutane < 1-Iodobutane < 1-Bromobutane
Which is the correct increasing order of boiling points of the following compounds?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane
Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane
1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene
1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Multiple Choice Questions (Type-II) Note: In the following questions two or more options may be correct.
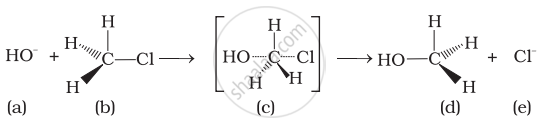
Which of the statements are correct about above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridised.
(iii) In (c) carbon atom is sp2 hybridised.
(iv) (a) and (e) both are electrophiles.
Which of the following statements are correct about this reaction?
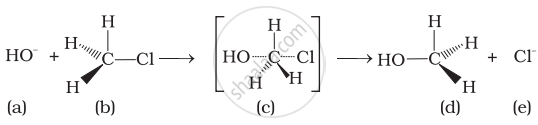
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
Which of the following statements are correct about the reaction intermediate?
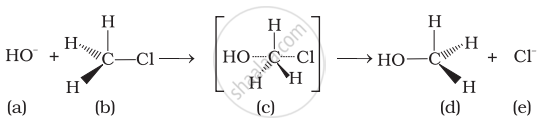
(i) Intermediate (c) is unstable because in this carbon is attached to 5 atoms.
(ii) Intermediate (c) is unstable because carbon atom is sp2 hybridised.
(iii) Intermediate (c) is stable because carbon atom is sp2 hybridised.
(iv) Intermediate (c) is less stable than the reactant (b).
Which of the following statements are correct about the mechanism of this reaction?
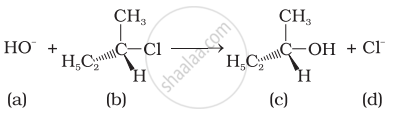
(i) A carbocation will be formed as an intermediate in the reaction.
(ii) \[\ce{OH-}\] will attach the substrate (b) from one side and \[\ce{Cl-}\] will leave it simultaneously from other side.
(iii) An unstable intermediate will be formed in which \[\ce{OH-}\] and \[\ce{Cl-}\] will be attached by weak bonds.
(iv) Reaction proceeds through SN1 mechanism.
Which of the following statements are correct about the kinetics of this reaction?
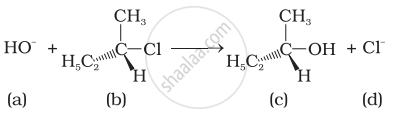
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Haloalkanes contain halogen atom (s) attached to the sp3 hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds.
(i) 2-Bromopentane
(ii) Vinyl chloride (chloroethene)
(iii) 2-chloroacetophenone
(iv) Trichloromethane
Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form same product on treatment with alcoholic KOH.
(ii) Both the compounds form same product on treatment with aq.NaOH.
(iii) Both the compounds form same product on reduction.
(iv) Both the compounds are optically active.
Which of the following compounds are gem-dihalides?
(i) Ethylidene chloride
(ii) Ethylene dichloride
(iii) Methylene chloride
(iv) Benzyl chloride
Which of the following are secondary bromides?
(i) \[\ce{(CH3)2 CHBr}\]
(ii) \[\ce{(CH3)3C CH2Br}\]
(iii) \[\ce{CH3CH(Br)CH2CH3}\]
(iv) \[\ce{(CH3)2 CBrCH2CH3}\]
Which of the following compounds can be classified as aryl halides?
(i) \[\ce{p-ClC6H4CH2CH(CH3)2}\]
(ii) \[\ce{p-CH3CHCl(C6H4)CH2CH3}\]
(iii) \[\ce{o-BrH2C - C6H4CH(CH3)CH2CH3}\]
(iv) \[\ce{C6H5 - Cl}\]
Alkyl halides are prepared from alcohol by treating with ______.
(i) HCl + ZnCl2
(ii) Red P + Br
(iii) H2SO4 + KI
(iv) All the above
Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of ______ or ______.
(i) CaF2
(ii) CoF2
(ii) Hg2F2
(iv) NaF
Short Answer Type
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
Out of o-and p-dibromobenzene which one has higher melting point and why?
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Why iodoform has appreciable antiseptic property?
Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Which of the following compounds (a) and (b) will not react with a mixture of \[\ce{NaBr}\] and \[\ce{H2SO4}\]. Explain why?
(a) \[\ce{CH3CH2CH2OH}\]
(b) 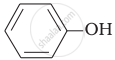
Which of the products will be major product in the reaction given below? Explain.
\[\ce{CH3CH = CH2 + HI -> \underset{(A)}{CH3CH2CH2I} + \underset{(B)}{CH3CHICH3}}\]
Why is the solubility of haloalkanes in water very low?
Draw other resonance structures related to the following structure and find out whether the functional group present in the molecule is ortho, para directing or meta directing.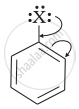
Classify the following compound as a primary, secondary and tertiary halide.
1-Bromobut-2-ene
Primary halide
Secondary halide
Tertiary halide
Classify the following compound as a primary, secondary and tertiary halide.
4-Bromopent-2-ene
Primary halide
Secondary halide
Tertiary halide
Classify the following compound as a primary, secondary and tertiary halide.
2-Bromo-2-methylpropane
Primary halide
Secondary halide
Tertiary halide
Compound ‘A’ with molecular formula \[\ce{C4H9Br}\] is treated with aq. \[\ce{KOH}\] solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. \[\ce{KOH}\] solution, the rate of reaction was found to be dependent on concentration of compound and \[\ce{KOH}\] both.
(i) Write down the structural formula of both compounds ‘A’ and ‘B’.
(ii) Out of these two compounds, which one will be converted to the product with inverted configuration.
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
Identify the products A and B formed in the following reaction:
\[\ce{CH3 - CH2 - CH = CH - CH3 + HCl -> A + B}\]
Which of the following compounds will have the highest melting point and why?
| (I) |  |
|
(II) |
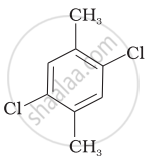 |
| (III) | 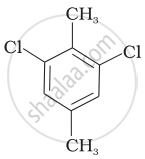 |
Write down the structure and IUPAC name for neo-pentylbromide.
A hydrocarbon of molecular mass 72 g mol–1 gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
Name the alkene which will yield 1-chloro-1-methylcyclohexane by its reaction with \[\ce{HCl}\]. Write the reactions involved.
Which of the following haloalkanes reacts with aqueous \[\ce{KOH}\] most easily? Explain giving reason.
1-Bromobutane
2-Bromobutane
2-Bromo-2-methylpropane
2-Chlorobutane
Why can aryl halides not be prepared by reaction of phenol with \[\ce{HCl}\] in the presence of \[\ce{ZnCl2}\]?
Which of the following compounds would undergo SN1 reaction faster and why?
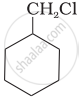 |
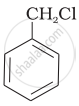 |
| (A) | (B) |
Allyl chloride is hydrolysed more readily than n-propyl chloride. Why?
Why is it necessary to avoid even traces of moisture during the use of a Grignard reagent?
How do polar solvents help in the first step in SN1 mechanism?
Write a test to detect the presence of double bond in a molecule.
Diphenyls are potential threat to the environment. How are these produced from arylhalides?
What are the IUPAC names of the insecticide DDT and benzene hexachloride? Why is their use banned in India and other countries?
Elimination reactions (especially β-elimination) are as common as the nucleophilic substitution reaction in case of alkyl halides. Specify the reagents used in both cases.
How will you obtain monobromobenzene from aniline?
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
| (I) | 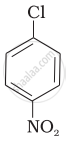 |
| (II) | 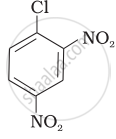 |
| (III) | 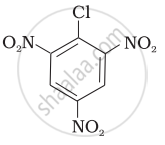 |
tert-Butylbromide reacts with aq. \[\ce{NaOH}\] by SN1 mechanism while n-butylbromide reacts by SN2 mechanism. Why?
Predict the major product formed when HCl is added to isobutylene. Explain the mechanism involved.
Discuss the nature of C – X bond in the haloarenes.
How can you obtain iodoethane from ethanol when no other iodine-containing reagent except NaI is available in the laboratory?
Cyanide ion acts as an ambident nucleophile. From which end it acts as a stronger nucleophile in aqueous medium? Give reason for your answer.
Matching Type Note: Match the items given in Column I and Column II in the following questions.
Match the compounds given in Column I with the effects given in Column II.
| Column I | Column II | |
| (i) | Chloramphenicol | (a) Malaria |
| (ii) | Thyroxine | (b) Anaesthetic |
| (iii) | Chloroquine | (c) Typhoid fever |
| (iv) | Chloroform | (d) Goiter |
| (e) Blood substituent |
Match the items of Column I and Column II.
| Column I | Column II | |
| (i) | SN1 reaction | (a) vic-dibromides |
| (ii) | Chemicals in fire extinguisher | (b) gem-dihalides |
| (iii) | Bromination of alkenes | (c) Racemisation |
| (iv) | Alkylidene halides | (d) Saytzeff rule |
| (v) | Elimination of HX from alkylhalide | (e) Chlorobromocarbons |
Match the structures of compounds given in Column I with the classes of compounds given in Column II.
| Column I | Column II | |
| (i) | \[\begin{array}{cc} \ce{CH3 - CH - CH3}\\ |\phantom{..}\\ \ce{X}\phantom{..} \end{array}\] |
(a) Aryl halide |
| (ii) | \[\ce{CH2 = CH - CH2 - X}\] | (b) Alkyl halide |
| (iii) | 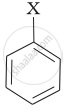 |
(c) Vinyl halide |
| (iv) | \[\ce{CH2 = CH - X}\] | (d) Allyl halide |
Match the reactions given in Column I with the types of reactions given in Column II.
| Column I | Column II | |
| (i) | 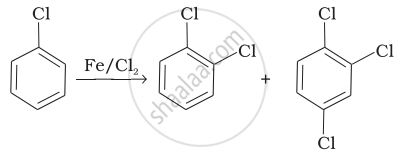 |
(a) Nucleophilic aromatic substitution |
| (ii) | \[\begin{array}{cc} \ce{CH3 - CH = CH2 + HBr -> CH3 - CH - CH3}\\ \phantom{............................}|\phantom{}\\ \phantom{.............................}\ce{Br}\phantom{} \end{array}\] |
(b) Electrophilic aromatic substitution |
| (iii) | 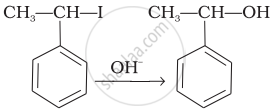 |
(c) Saytzeff elimination |
| (iv) | 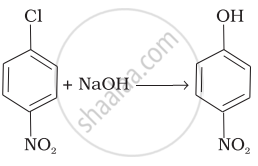 |
(d) Electrophilic addition |
| (v) | \[\begin{array}{cc} \ce{CH3 CH2 CH CH3 ->[alc.KOH] CH3 CH = CH CH3}\\ \phantom{}|\phantom{..........................}\\ \phantom{}\ce{Br}\phantom{........................} \end{array}\] |
(e) Nucleophilic substitution (SN1) |
Match the structures given in Column I with the names in Column II.
| Column I | Column II | |
| (i) | 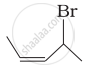 |
(a) 4-Bromopent-2-ene |
| (ii) | 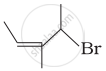 |
(b) 4-Bromo-3-methylpent-2-ene |
| (iii) | 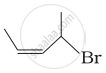 |
(c) 1-Bromo-2-methylbut-2-ene |
| (iv) | 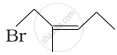 |
(d) 1-Bromo-2-methylpent-2-ene |
Match the reactions given in Column I with the names given in Column II.
| Column I | Column II | |
| (i) | 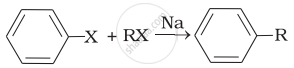 |
(a) Fittig reaction |
| (ii) | 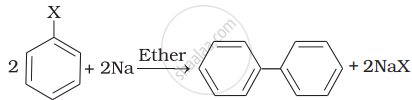 |
(b) Wurtz Fittig reaction |
| (iii) | 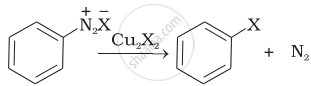 |
(c) Finkelstein reaction |
| (iv) | \[\ce{C2H5Cl + Nal ->[dry acetone] C2H5l + NaCl}\] | (d) Sandmeyer reaction |
Assertion and Reason Type Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Phosphorus chlorides (tri and penta) are preferred over thionyl chloride for the preparation of alkyl chlorides from alcohols.
Reason: Phosphorus chlorides give pure alkyl halides.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: The boiling points of alkyl halides decrease in the order:
\[\ce{RI > RBr > RCl > RF}\]
Reason: The boiling points of alkyl chlorides, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: KCN reacts with methyl chloride to give methyl isocyanide.
Reason: CN– is an ambident nucleophile.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: tert-Butyl bromide undergoes Wurtz reaction to give 2, 2, 3, 3-tetramethylbutane.
Reason: In Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion: Presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution.
Reason: Nitro group, being an electron-withdrawing group decreases the electron density over the benzene ring.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: In monohaloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason: Halogen atom is a ring deactivator.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: Aryl iodides can be prepared by reaction of arenes with iodine in the presence of an oxidising agent.
Reason: Oxidising agent oxidises I2 into HI.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: It is difficult to replace chlorine by –OH in chlorobenzene in comparison to that in chloroethane.
Reason: Chlorine-carbon \[\ce{(C - Cl)}\] bond in chlorobenzene has a partial double bond character due to resonance.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: Hydrolysis of (–)-2-bromooctane proceeds with inversion of configuration.
Reason: This reaction proceeds through the formation of a carbocation.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Assertion: Nitration of chlorobenzene leads to the formation of m-nitrochlorobenzene.
Reason: –NO2 group is a m-directing group.
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Long Answer Type
Some alkylhalides undergo substitution whereas some undergo elimination reaction on treatment with bases. Discuss the structural features of alkyl halides with the help of examples which are responsible for this difference.
Some halogen containing compounds are useful in daily life. Some compounds of this class are responsible for exposure of flora and fauna to more and more of UV light which causes destruction to a great extent. Name the class of these halocompounds. In your opinion, what should be done to minimise harmful effects of these compounds.
Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
Solutions for 10: Haloalkanes and Haloarenes
![NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 10 - Haloalkanes and Haloarenes NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 10 - Haloalkanes and Haloarenes - Shaalaa.com](/images/chemistry-english-class-12_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
NCERT Exemplar solutions for Chemistry [English] Class 12 chapter 10 - Haloalkanes and Haloarenes
Shaalaa.com has the CBSE Mathematics Chemistry [English] Class 12 CBSE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT Exemplar solutions for Mathematics Chemistry [English] Class 12 CBSE 10 (Haloalkanes and Haloarenes) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. NCERT Exemplar textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] Class 12 chapter 10 Haloalkanes and Haloarenes are Introduction of Haloalkanes and Haloarenes, Nomenclature, Nature of C-X Bond, Physical Properties of Haloalkanes and Haloarenes, Reactions of Haloalkanes - Nucleophilic Substitution Reactions, R-s and D-l Configuration, Reactions of Haloarenes - Nucleophilic Substitution, Polyhalogen Compounds, Reactions of Haloarenes - Reaction with Metals, Reactions of Haloalkanes - Elimination Reactions, Classification of Haloalkanes and Haloarenes, Reactions of Haloalkanes - Reaction with Metals, Reactions of Haloarenes - Electrophilic Substitution Reactions, Haloalkanes and Haloarenes Numericals, Methods of Preparation of Haloalkanes, Methods of Preparation of Haloarenes.
Using NCERT Exemplar Chemistry [English] Class 12 solutions Haloalkanes and Haloarenes exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in NCERT Exemplar Solutions are essential questions that can be asked in the final exam. Maximum CBSE Chemistry [English] Class 12 students prefer NCERT Exemplar Textbook Solutions to score more in exams.
Get the free view of Chapter 10, Haloalkanes and Haloarenes Chemistry [English] Class 12 additional questions for Mathematics Chemistry [English] Class 12 CBSE, and you can use Shaalaa.com to keep it handy for your exam preparation.