Advertisements
Advertisements
प्रश्न
Which is the correct increasing order of boiling points of the following compounds?
1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane
पर्याय
Butane < 1-Chlorobutane < 1-Bromobutane < 1-Iodobutane
1-Iodobutane < 1-Bromobutane < 1-Chlorobutane < Butane
Butane < 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane
Butane < 1-Chlorobutane < 1-Iodobutane < 1-Bromobutane
उत्तर
Butane < 1-Chlorobutane < 1-Bromobutane < 1-Iodobutane
Explanation:
The higher the surface area, the higher will be the intermolecular forces of attraction and thus boiling point too. Boiling point increases with increase in molecular mass of halogen atom for the similar type of alkyl halide. Butane has no halogen atom and rest of all three compounds are halo derivatives of butane.
Atomic mass of iodine is highest so the boiling point of 1-iodobutane is maximum among all the given compounds.
APPEARS IN
संबंधित प्रश्न
p-Dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss.
Which of the following compounds has the highest boiling point?
How many structural isomers are possible for a compound with the molecular formula C3H7Cl?
Which of the following halide is 2°?
p-dichlorobenzene has a higher melting point than its o- and m-isomers because ____________.
Arrange the following compounds in the increasing order of their densities.
(a)
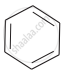
(b)
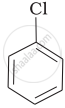
(c)
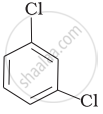
(d)
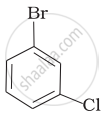
Which of the following compounds will have the highest melting point and why?
| (I) |  |
|
(II) |
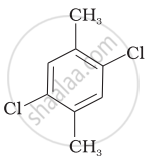 |
| (III) | 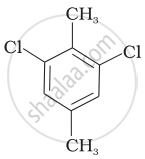 |
Which out of the following is an intensive property?
Arrange the following compounds in increasing order of their boiling points:
CH3CH2OH, CH3−CHO, CH3−COOH
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
