Advertisements
Advertisements
प्रश्न
Assertion: KCN reacts with methyl chloride to give methyl isocyanide.
Reason: CN– is an ambident nucleophile.
पर्याय
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct but reason is wrong statement.
Assertion is wrong but reason is correct statement.
Assertion and reason both are correct statements but reason is not correct explanation of assertion.
उत्तर
Assertion is wrong but reason is correct statement.
Explanation:
\[\ce{\underset{Alkyl cyanide}{R - Cl + KCN –> R - CN + KCl}}\]
Isocyanide is not obtained in this reaction. CN– is an ambident nucleophile.
APPEARS IN
संबंधित प्रश्न
How do you convert the following:
Ethanol to propanenitrile
Which compound in the following pair will react faster in SN2 reaction with OH−?
(CH3)3CCl or CH3Cl
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
What is the action of the following on ethyl bromide
alcoholic solution of potassium hydroxide.
Which one is most reactive towards SN1 reaction?
2-Bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is ____________.
In which reaction mechanism carbocation is formed?
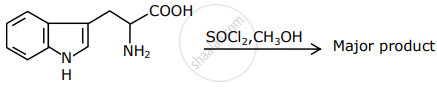
The major product formed in the following reaction is:
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
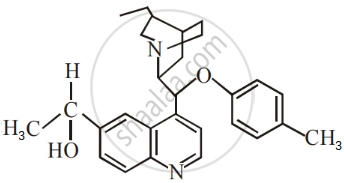
The number of chiral carbons present in the molecule given below is ______.