Advertisements
Advertisements
प्रश्न
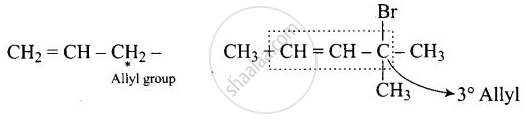
The position of \[\ce{-Br}\] in the compound in \[\ce{CH3CH = CH(Br)(CH3)2}\] can be classified as ______.
पर्याय
Allyl
Aryl
Vinyl
Secondary
उत्तर
The position of \[\ce{-Br}\] in the compound in \[\ce{CH3CH = CH(Br)(CH3)2}\] can be classified as Allyl.
Explanation:
It is allylic compound in which Br is attached next to double bonded carbon.
APPEARS IN
संबंधित प्रश्न
Name the following halide according to the IUPAC system and classify it as an alkyl, allyl, benzoyl (primary, secondary, tertiary), vinyl or aryl halide:
CH3CH2CH(CH3)CH(C2H5)Cl
Name the following halide according to the IUPAC system and classify it as an alkyl, allyl, benzoyl (primary, secondary, tertiary), vinyl or aryl halide:
p-ClC6H4CH2CH(CH3)2
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
CH3C(C2H5 )2CH2Br
Name the following halide according to the IUPAC system and classify it as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halide:
CH3C(Cl)(C2H5)CH2CH3
Name the following halide according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3C(Cl)(C2H5)CH2CH3}\]
Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3CH2C(CH3)2CH2I}\]
Name the following halide according to IUPAC system and classify as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halide:
\[\ce{CH3C(C2H5)2CH2Br}\]
Give the IUPAC name of the compound.
\[\ce{CICH2 C ≡ CCH2 Br}\]
Draw the structure of major monohalo products in the following reaction:
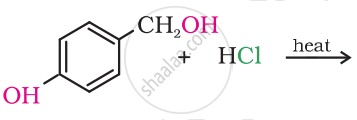
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
