Advertisements
Advertisements
Question
Explain the following reaction:
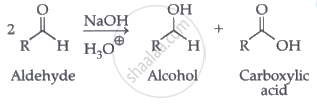
Cannizzaro reaction
Solution
Aldehydes which do not have an α-hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on heating with concentrated alkali. In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
APPEARS IN
RELATED QUESTIONS
Write the chemical equation for the reaction involved in Cannizzaro reaction.
An organic compound with the molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1, 2-benzenedicarboxylic acid. Identify the compound.
Write the equations involved in the following reactions:
Etard reaction
Complete the following reactions:
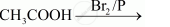
Write the product formed when p-nitro chlorobenzene is heated with aqueous NaOH at 443K followed by acidification?
complete the following reaction:
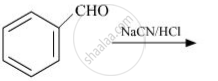
complete the following reaction:
\[\begin{array}{cc}
\phantom{...}\ce{CH3} \\
| \\
\phantom{.................}\ce{CH3-CH-COOH ->[(i) Br2/Red P4][(ii)H2O]}
\end{array}\]
Complete the following reaction:

The key step in cannizzaro reaction in the inter molecular shift qf
\[\begin{array}{cc}
\ce{D}\phantom{........................}\\
|\phantom{.........................}\\
\ce{2D - C = O + OH^- ->[Cannizzaro] X and Y}
\end{array}\]
(Y is alcohol, D is deuterium)
X and Y will have the structure:
