Advertisements
Advertisements
Question
Explain the following reactions:
Wolff Kishner reduction
Solution
In this reaction, the reduction of carbonyl compounds to hydrocarbons takes place by heating them with hydrazine and a base to form hydrazine which is further reduced to form a methylene group along with nitrogen gas.
\[\begin{array}{cc}
\phantom{..}\ce{R}\phantom{.........................}\ce{R}\phantom{.......................}\ce{R}\phantom{................}\\
\phantom{..}\backslash\phantom{.........................}\backslash\phantom{........................}\backslash\phantom{.............}\\
\phantom{....}\ce{C = \underset{Hydrazine}{O + H2N - NH2} -> C = N - NH2 ->[glycol + KOH][180°C] CH2 + N2(g)}\\
\phantom{..}/\phantom{.........................}/\phantom{........................}/\phantom{.............}\\
\phantom{.........}\ce{\underset{Carbonyl compound}{R'}}\phantom{..................}\ce{R'}\phantom{...................}\ce{\underset{Hydrocarbon}{R'}}\phantom{........................}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
How will you bring about the following conversions?
Propanone to propane
Write the chemical reaction involved in Wolff-Kishner reduction.
Predict the products of the following reactions :
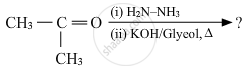
Write the structures of the main products when acetone (CH3 − CO − CH3) reacts with the following reagent :
Zn − Hg/conc. HCl
Predict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.
Zinc amalgam and dilute hydrochloric acid
Predict the main product of the following reaction:\[\begin{array}{c}
\ce{O\phantom{----}O\phantom{-}}\\
\ce{||\phantom{----}||\phantom{-}}\\
\ce{CH3-C-CH2-C-OCH3}
\end{array}\ce{->[(i)NaBH4][(ii)H+]}\]
Write chemical equation of the following reaction :
Acetophenone is treated with `("Zn"("Hg"))/"Conc.HCl"`.
Reduction of > C = O to CH2 can be carried out with
The product formed in the following chemical reaction is:
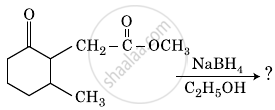
Write the main product in the following reaction:
\[\ce{CH3CH2CHO ->[Zn(Hg)/Conc. HCl]}\]
