Advertisements
Advertisements
Question
Arrange the following in the increasing order of their property indicated:
4-Nitrobenzoic acid, benzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxy benzoic acid (Acid strength)
Solution
The increasing order of acidic strength of the following compounds is 4-methoxy benzoic acid < benzoic acid < 4-nitrobenzoic acid < 3,4-dinitrobenzoic acid. (Due to the presence of electron withdrawing group).
APPEARS IN
RELATED QUESTIONS
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
Butan-1-ol
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Intermolecular hydrogen bonding is strongest in ______.
Acidity of phenol is due to ____________.
Strength of acidity is in order:
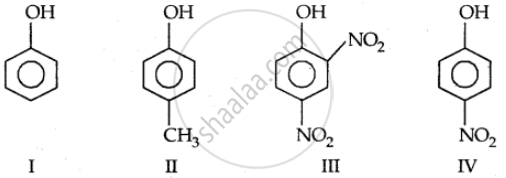
Which of the following compounds is most acidic?
Arrange the following in decreasing order of acidic character:
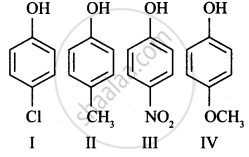
In the following compounds:
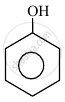 |
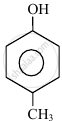 |
 |
 |
| (I) | (II) | (III) | (IV) |
The order to acidity is ______.
Which one of the following has the lowest pKa value?
Compare acidity of phenol with that of ethanol.
