Advertisements
Advertisements
Question
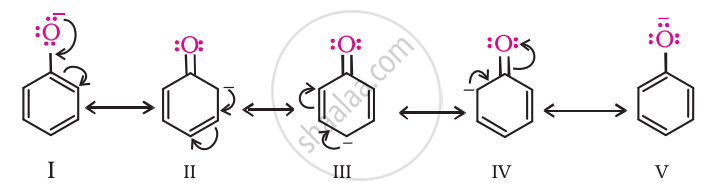
Compare acidity of phenol with that of ethanol.
Solution
Phenol is more acidic than ethanol. This is because the phenoxide ion obtained after the release of a proton from phenol becomes stable through resonance, whereas the ethoxide ion (after the release of a proton from ethanol) is not stable.
APPEARS IN
RELATED QUESTIONS
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
Butan-1-ol
Intermolecular hydrogen bonding is strongest in ______.
Phenol is more acidic than alcohol because ____________.
In CH3CH2OH, the bond that undergoes heterolytical change most readily is ____________.
Phenol reacts with Br2 in CS2 at low temperature to give ____________.
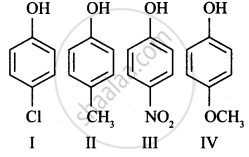
Which of the following compounds is most acidic?
Arrange the following in decreasing order of acidic character:
Which of the following statements is true:
Mark the correct order of decreasing acid strength of the following compounds.
| (a) | 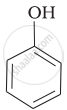 |
| (b) | 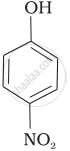 |
| (c) | 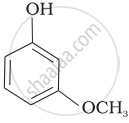 |
| (d) | 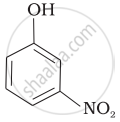 |
| (e) | 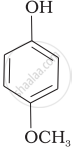 |
Which is the final product ‘A’ (major) in the given reaction?
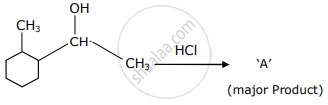
For the pair phenol and cyclohexanol, answer the following:
Why is phenol more acidic than cyclohexanol?
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
2-Methylbutan-2-ol
Give the structure of the product you would expect when the following alcohol reacts with HBr.
Butan-1-ol
Give the structure of the product you would expect when the following alcohol reacts with HBr.
2-Methylbutan-2-ol
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
2-Methylbutan-2-ol
