Advertisements
Advertisements
Question
Give the structure of the product you would expect when the following alcohol reacts with HBr.
2-Methylbutan-2-ol
Solution
\[\begin{array}{cc}
\phantom{.}\ce{CH3}\phantom{...........................}\ce{CH3}\phantom{.........}\\
|\phantom{..............................}|\phantom{...........}\\
\ce{CH3 - C - CH2CH3 + HBr->[\Delta]CH3 - C - CH2CH3 + H2O}\\
|\phantom{..............................}|\phantom{...........}\\
\phantom{.}\ce{OH}\phantom{....................}\ce{\underset{2-bromo-2-methylbutane}{Br}}\phantom{..}\
\end{array}\]
RELATED QUESTIONS
Give two reactions that show the acidic nature of phenol.
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Intermolecular hydrogen bonding is strongest in ______.
In the following compounds:
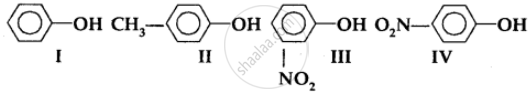
The order of acidity is
Arrange the following in decreasing order of acidic character:
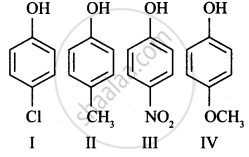
Assertion: o-Nitrophenol is less soluble in water than the m- and p-isomers.
Reason: m- and p- Nitrophenols exist as associated molecules.
Phenol is used in the manufacture of
Arrange the following in the increasing order of their property indicated:
4-Nitrobenzoic acid, benzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxy benzoic acid (Acid strength)
In the following compounds:
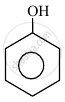 |
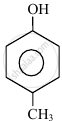 |
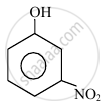 |
 |
| (I) | (II) | (III) | (IV) |
The order to acidity is ______.
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
2-Methylbutan-2-ol
