Advertisements
Advertisements
प्रश्न
Give the structure of the product you would expect when the following alcohol reacts with HBr.
2-Methylbutan-2-ol
उत्तर
\[\begin{array}{cc}
\phantom{.}\ce{CH3}\phantom{...........................}\ce{CH3}\phantom{.........}\\
|\phantom{..............................}|\phantom{...........}\\
\ce{CH3 - C - CH2CH3 + HBr->[\Delta]CH3 - C - CH2CH3 + H2O}\\
|\phantom{..............................}|\phantom{...........}\\
\phantom{.}\ce{OH}\phantom{....................}\ce{\underset{2-bromo-2-methylbutane}{Br}}\phantom{..}\
\end{array}\]
संबंधित प्रश्न
Account for the following:
o-nitrophenol is more steam volatile than p-nitrophenol.
Intermolecular hydrogen bonding is strongest in ______.
Phenol is more acidic than alcohol because ____________.
Acidity of phenol is due to ____________.
Which of the following compounds is most acidic?
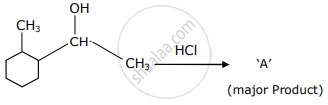
Which is the final product ‘A’ (major) in the given reaction?
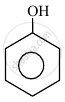
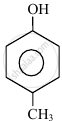
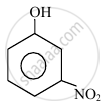
In the following compounds:
 |
 |
 |
 |
| (I) | (II) | (III) | (IV) |
The order to acidity is ______.
Which one of the following has the lowest pKa value?
Give the structure of the product you would expect when the following alcohol reacts with HBr.
Butan-1-ol
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
2-Methylbutan-2-ol
