Advertisements
Advertisements
Question
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Solution
Phenol is considered to be a resonance hybrid of the following structures:
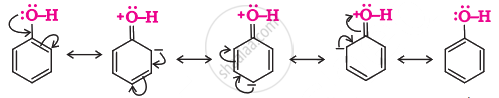
The +R effect of the –OH group increases the electron density on the benzene ring, which makes the attack of the electrophile easier. Hence, the presence of the –OH group activates the benzene ring towards electrophilic substitution reactions. Since the electron density at the ortho and para positions is relatively high, electrophilic substitution is mainly more at the ortho and para positions.
APPEARS IN
RELATED QUESTIONS
The product obtained from the reaction is:
Phenols do not react with one of the following:
Phenol is more acidic than alcohol because ____________.
Acidity of phenol is due to ____________.
The ionization constant of phenol is higher than that of ethanol because ____________.
In the following compounds:
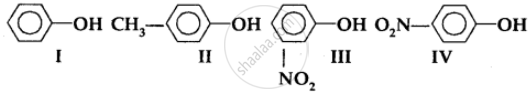
The order of acidity is
Strength of acidity is in order:
Which of the following compounds is most acidic?
Arrange the following in decreasing order of acidic character:
Which of the following statements is true:
Out of o-nitrophenol and o-cresol which is more acidic?
Phenol is used in the manufacture of
Arrange the following in the increasing order of their property indicated:
4-Nitrobenzoic acid, benzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxy benzoic acid (Acid strength)
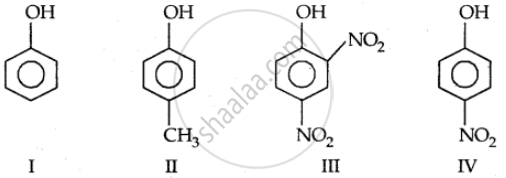
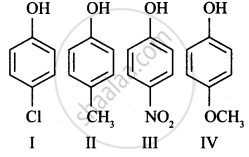
In the following compounds:
 |
 |
 |
 |
| (I) | (II) | (III) | (IV) |
The order to acidity is ______.
For the pair phenol and cyclohexanol, answer the following:
Why is phenol more acidic than cyclohexanol?
Give the structure of the product you would expect when the following alcohol reacts with HBr.
Butan-1-ol
Give the structure of the product you would expect when the following alcohol reacts with HBr.
2-Methylbutan-2-ol
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
Butan-1-ol
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
2-Methylbutan-2-ol
