Advertisements
Advertisements
Question
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
Butan-1-ol
Solution
\[\ce{\underset{Butan-1-ol}{CH3CH2CH2CH2OH} + SOCl2 ->[\Delta]\underset{1-chlorobutane}{CH3CH2CH2CH2Cl} + SO2 + HCl}\]
RELATED QUESTIONS
Give the structure of the product you would expect when the following alcohol reacts with HCl–ZnCl2.
Butan-1-ol
Intermolecular hydrogen bonding is strongest in ______.
Phenols do not react with one of the following:
The ionization constant of phenol is higher than that of ethanol because ____________.
In CH3CH2OH, the bond that undergoes heterolytical change most readily is ____________.
Strength of acidity is in order:
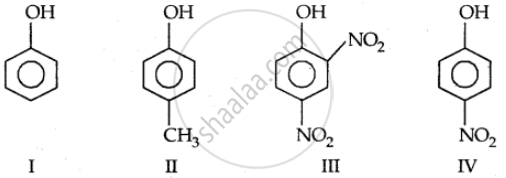
Which one of the following compounds has the most acid nature?
Which of the following statements is true:
For the pair phenol and cyclohexanol, answer the following:
Why is phenol more acidic than cyclohexanol?
Compare acidity of phenol with that of ethanol.
