Advertisements
Advertisements
प्रश्न
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
Butan-1-ol
उत्तर
\[\ce{\underset{Butan-1-ol}{CH3CH2CH2CH2OH} + SOCl2 ->[\Delta]\underset{1-chlorobutane}{CH3CH2CH2CH2Cl} + SO2 + HCl}\]
संबंधित प्रश्न
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
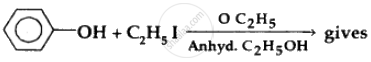
The product obtained from the reaction is:
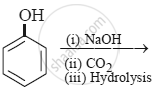
In the following compounds:
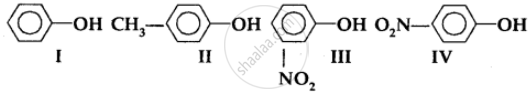
The order of acidity is
Phenol reacts with Br2 in CS2 at low temperature to give ____________.
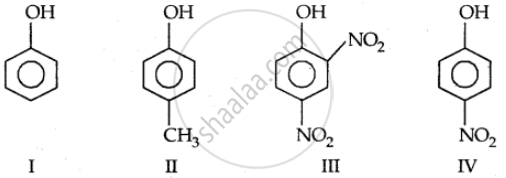
Strength of acidity is in order:
Which of the following compounds is most acidic?
Arrange the following in decreasing order of acidic character:
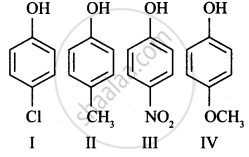
Mark the correct order of decreasing acid strength of the following compounds.
| (a) | 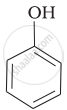 |
| (b) | 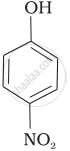 |
| (c) | 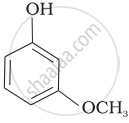 |
| (d) | 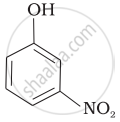 |
| (e) | 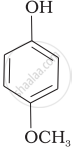 |
Out of o-nitrophenol and o-cresol which is more acidic?
