Advertisements
Advertisements
Question
Give the structure of the product you would expect when the following alcohol reacts with HBr.
Butan-1-ol
Solution
\[\ce{\underset{Butan-1-ol}{CH3CH2CH2CH2OH} + HBr->[\Delta]\underset{1-bromobutane}{CH3CH2CH2CH2Br} + H2O}\]
RELATED QUESTIONS
Write the equation involved in the acetylation of Salicylic acid.
Give two reactions that show the acidic nature of phenol.
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Intermolecular hydrogen bonding is strongest in ______.
Phenol is more acidic than alcohol because ____________.
In the following compounds:
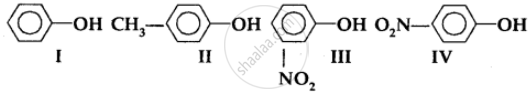
The order of acidity is
In CH3CH2OH, the bond that undergoes heterolytical change most readily is ____________.
Which of the following statements is true:
Mark the correct order of decreasing acid strength of the following compounds.
| (a) | 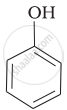 |
| (b) | 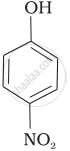 |
| (c) | 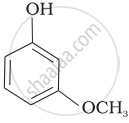 |
| (d) | 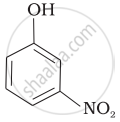 |
| (e) | 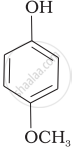 |
Assertion: o-Nitrophenol is less soluble in water than the m- and p-isomers.
Reason: m- and p- Nitrophenols exist as associated molecules.
