Advertisements
Advertisements
प्रश्न
Compare acidity of phenol with that of ethanol.
उत्तर
Phenol is more acidic than ethanol. This is because the phenoxide ion obtained after the release of a proton from phenol becomes stable through resonance, whereas the ethoxide ion (after the release of a proton from ethanol) is not stable.
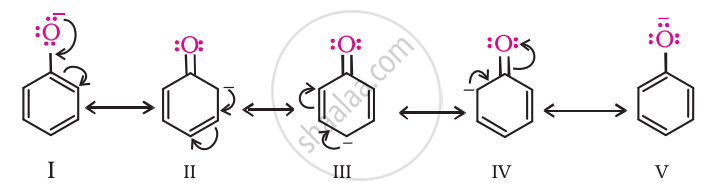
APPEARS IN
संबंधित प्रश्न
Write the equation involved in the acetylation of Salicylic acid.
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Intermolecular hydrogen bonding is strongest in ______.
Phenols do not react with one of the following:
The ionization constant of phenol is higher than that of ethanol because ____________.
In the following compounds:
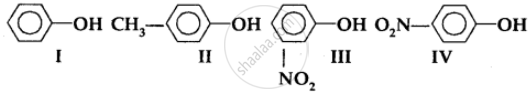
The order of acidity is
In CH3CH2OH, the bond that undergoes heterolytical change most readily is ____________.
Which of the following compounds is most acidic?
Which one of the following compounds has the most acid nature?
Arrange the following in decreasing order of acidic character:
Mark the correct order of decreasing acid strength of the following compounds.
| (a) | 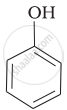 |
| (b) | 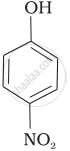 |
| (c) | 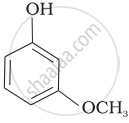 |
| (d) | 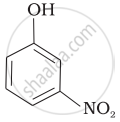 |
| (e) | 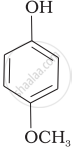 |
Out of o-nitrophenol and o-cresol which is more acidic?
Phenol is used in the manufacture of
Arrange the following in the increasing order of their property indicated:
4-Nitrobenzoic acid, benzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxy benzoic acid (Acid strength)
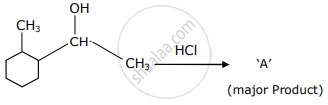
Which is the final product ‘A’ (major) in the given reaction?
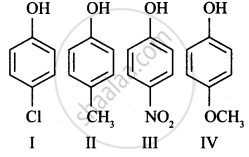
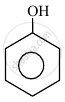
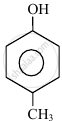
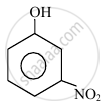
In the following compounds:
 |
 |
 |
 |
| (I) | (II) | (III) | (IV) |
The order to acidity is ______.
