Advertisements
Advertisements
Question
Observe the graph shown in figure and answer the following questions:
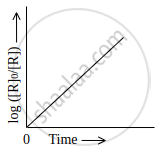
- What is the order of the reaction?
- What is the slope of the curve?
- Write the relationship between k and t1/2 (half life period).
Solution
- The order of the reaction is first-order reaction.
- Slope of the curve = `k/2.303`
- The half-life for the first-order reaction is given by t1/2 = `0.693/k`
APPEARS IN
RELATED QUESTIONS
The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308 K. If the value of A is 4 × 1010 s−1. Calculate k at 318 K and Ea.
Define order of reaction. How does order of a reaction differ from molecularity for a complex reaction?
Which of the following graphs is correct for a first order reaction?

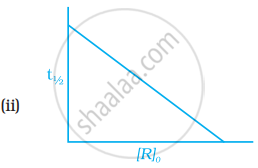
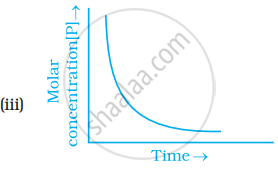
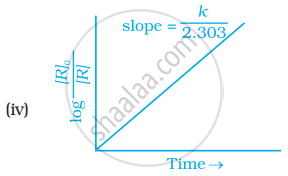
The rate constant of a first order reaction is 6.9 × 10–3s–1. How much time will it take to reduce the initial concentration to its 1/8th value?
First order reaction is 50% complete in 1.26 × 1014s. How much time could it take for 100% completion?
The reaction X → product
Follow first order of kinetics. In 40 minutes the concentration of 'X' changes from 0.1 m to 0.025. M. The rate of reaction when concentration of X is 0.01 m is.
The decomposition of formic acid on gold surface follows first-order kinetics. If the rate constant at 300 K is 1.0 × 10−3 s−1 and the activation energy Ea = 11.488 kJ mol−1, the rate constant at 200 K is ______ × 10−5 s−1. (Round off to the Nearest Integer)
(Given R = 8.314 J mol−1 K−1)
For a first order reaction, the ratio of the time for 75% completion of a reaction to the time for 50% completion is ______. (Integer answer)
The slope in the plot of ln[R] vs. time for a first order reaction is ______.
What is the rate constant?
