Advertisements
Advertisements
Question
Which of the following species cannot act as a ligand? Give reason.
Options
OH−
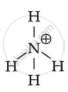
`"NH"_4^+`
CH3NH2
H2O
Solution
`bb("NH"_4^+)`
Explanation:
Because it is unable of providing a core metal ion with a lone pair of electrons.
APPEARS IN
RELATED QUESTIONS
Explain the following, giving two examples:
Coordination entity
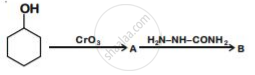
Write structures of compounds A and B of the following reaction :
Ligand (en) is an example of ___________.
When 1 mol \[\ce{CrCl3.6H2O}\] is treated with excess of \[\ce{AgNO3}\], 3 mol of \[\ce{AgCl}\] are obtained. The formula of the complex is ______.
Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand.
(i) It is a neutral ligand.
(ii) It is a didentate ligand.
(iii) It is a chelating ligand.
(iv) It is a unidentate ligand.
Match the complex ions given in Column I with the colours given in Column II and assign the correct code:
| Column I (Complex ion) | Column II (Colour) |
| A. \[\ce{[Co(NH3)6]^{3+}}\] | 1. Violet |
| B. \[\ce{[Ti(H2O)6]^{3+}}\] | 2. Green |
| C. \[\ce{[Ni(H2O)6]^{2+}}\] | 3. Pale blue |
| D. \[\ce{(Ni(H2O)4 (en)]^{2+} (aq)}\] | 4. Yellowish orange |
| 5. Blue |
Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code:
| Column I (Coordination Compound) | Column II (Central metal atom) |
| A. Chlorophyll | 1. rhodium |
| B. Blood pigment | 2. cobalt |
| C. Wilkinson catalyst | 3. calcium |
| D. Vitamin B12 | 4. iron |
| 5. magnesium |
Why chelate complexes are more stable than complexes with unidentate ligands?
Give two examples of unidentate ligand.
Give two examples of didentate ligand.
