Advertisements
Advertisements
Question
Explain the following, giving two examples:
Coordination number
Solution
The coordination number (CN) of a metal ion in a complex can be defined as the number of ligand donor atoms to which the metal is directly bonded. For example, in the complex ions, [PtCl6]2− and [Ni(NH3)4]2+, the coordination numbers of Pt and Ni are 6 and 4, respectively. Similarly, in the complex ions, [Fe(C2O4)3]3− and [Co(en)3]3+, the coordination number of both Fe and Co is 6 because \[\ce{C2O^{2−}4}\] and en (ethane-1, 2-diamine) are didentate ligands.
RELATED QUESTIONS
Explain the following, giving two examples:
Coordination entity
Amongst the following, the most stable complex is:
Write the structures of compounds A, B and C in the following reactions:
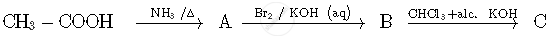
The oxidation number of Fe in K4[Fe(CN)6] is ____________.
A coordination compound \[\ce{CrCl3.4H2O}\] precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write structural formula of the compound and name it.
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
Give two examples of unidentate ligand.
What is meant by ambidentate ligand?
