Advertisements
Advertisements
Question
What is a chelate complex? Give one example.
Solution
When a di- or polydentate ligand uses its two or more donor atoms simultaneously to bind a single metal ion, it is said to be a chelate ligand. Such complexes are called chelate complexes.
For example: [CoCl2(en)2]+
APPEARS IN
RELATED QUESTIONS
Explain the following, giving two examples:
Coordination entity
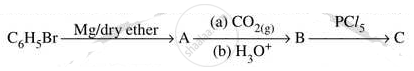
Write structures of compounds A, B and C in of the following reactions
Which of the following represents a chelate ligand?
Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code:
| Column I (Coordination Compound) | Column II (Central metal atom) |
| A. Chlorophyll | 1. rhodium |
| B. Blood pigment | 2. cobalt |
| C. Wilkinson catalyst | 3. calcium |
| D. Vitamin B12 | 4. iron |
| 5. magnesium |
The oxidation number d-arbitral occupation and co-ordination number of Cr in the complex cis [Cr(en)2Cl2]Cl are respectively.
Which of the following ligands can exhibit linkage isomerism?
Metal attached with EDTA in an octahedral complex, has coordination number ______.
Glycinato ligand is ______.
Assertion (A): EDTA is a hexadentate ligand.
Reason (R): EDTA has 2 nitrogen and 4 oxygen donor atoms.
What is meant by the chelate effect? Give an example.
