Advertisements
Advertisements
Question
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
Solution
Copper ferrocyanide
APPEARS IN
RELATED QUESTIONS
Classify the following ligands into monodentate and polydentate —
- Ammonia
- Carbon monoxide
- Ethylene diamine
- Ethylene diamine tetra acetate ion
Explain the following, giving two examples:
Coordination entity
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
(NH4)2[CoF4]
Write IUPAC names of the following compounds
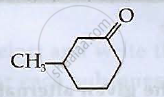
Write IUPAC names of the following compounds:
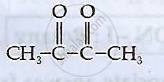
Write structures of compounds A, B and C in of the following reactions
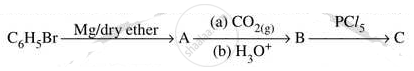
Write IUPAC name of the following Complex [Cr(NH3)3Cl3]
Write the IUPAC name of the following complex : [Co(NH3)5(CO3)]Cl.
Write the structures of compounds A, B and C in the following reactions:
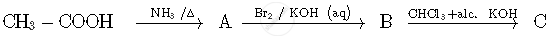
A group of atoms can function as a ligand only when:
The coordination number of the central ion may be obtained from:
When 0.1 mol \[\ce{CoCl3 (NH3)5}\] is treated with excess of \[\ce{AgNO3}\], 0.2 mol of \[\ce{AgCl}\] are obtained. The conductivity of solution will correspond to ______.
When 1 mol \[\ce{CrCl3.6H2O}\] is treated with excess of \[\ce{AgNO3}\], 3 mol of \[\ce{AgCl}\] are obtained. The formula of the complex is ______.
The correct \[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2Cl2]}\] is ______.
Arrange the following complexes in the increasing order of conductivity of their solution:
[Co(NH3)3Cl3], [Co(NH3)4Cl2]Cl, [Co(NH3)6]Cl3, [Cr(NH3)5Cl]Cl2
A coordination compound \[\ce{CrCl3.4H2O}\] precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write structural formula of the compound and name it.
In which of the following compounds, the oxidation number of iodine is fractional?
Which of the following is an ionic ligand?
The oxidation state of Fe in the brown ring complex [F3(H2O)5NO]SO4 is
The co-ordinate number and the oxidation state of the element E in the complex [E(en)2(C2O4)]NO2 are respectively?
The complex which has no d electrons in the central atom is:-
According to IUPAC nomenclatures, sodium nitroprusside is named as
The equivalents of ethylene diamine required to replace the neutral ligands from the coordination sphere of the trans-complex of CoCl3.4NH3 is ______. (Round off to the Nearest Integer).
Which of the following species cannot act as a ligand? Give reason.
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Homoleptic
What is meant by didentate ligand?
