Advertisements
Advertisements
Question
Natalite is a mixture of
(a) diethyl ether and methanol
(b) diethyl ether and ethanol
(c) dimethyl ether and methanol
(d) dimethyl ether and ethanol
Solution
diethyl ether and ethanol
APPEARS IN
RELATED QUESTIONS
What is metamerism?
Write the IUPAC name of the given compound:
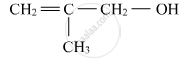
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{........................}\ce{CH2OH}\\
\phantom{..................}|\\
\ce{CH3 - CH - CH2 - CH - CH - CH3}\\
\phantom{}|\phantom{.............}|\phantom{........}\\
\phantom{..}\ce{CH3}\phantom{..........}\ce{OH}\phantom{........}
\end{array}\]
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{H2C = CH - CH - CH2 - CH2 - CH3}\\
|\phantom{..........}\\
\ce{OH}\phantom{........}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{H3C - CH - CH2 - CH - CH - CH2 - CH3}\\
\phantom{}|\phantom{.............}|\phantom{......}|\phantom{.........}\\
\phantom{}\ce{OH}\phantom{..........}\ce{OH}\phantom{...}\ce{C2H5}\phantom{......}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
|\phantom{......}|\phantom{..}\\
\ce{OH}\phantom{...}\ce{OH}\phantom{}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{HO - CH2 - CH - CH2 - OH}\\
|\phantom{..}\\
\ce{OH}
\end{array}\]
Write IUPAC name of the following compound:
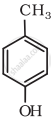
Write IUPAC name of the following compound:
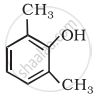
Write IUPAC name of the following compound:
C6H5 – O – C2H5
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH2 - O - CH - CH2 - CH3}\\
\phantom{...}|\\
\phantom{.....}\ce{CH3}
\end{array}\]
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
Give IUPAC name of the following ether:
\[\begin{array}{cc}
\ce{C2H5OCH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
Give IUPAC name of the following ether:
O2N – C6H4 – OCH3(p)
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
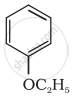
Give IUPAC name of the following ether:
Which of the following compounds is NOT prepared by the action of alcoholic NI3 on alkyl halide?
(a) CH3NH2
(b) CH3- CH2- NH2
(c) CH3 - CH2 - CH2 - NH2
(d) (CH3)3 C- NH2
Write the structure and IUPAC name of 'methyl-n-propyl ether'.
What is the action of hot HI on it?
3-Methylbutane-2-ol on heating with HI gives ______
How is phenol converted into the following?
benzoquinone
How is phenol converted into the following?
picric acid
Write IUPAC name of the following compound (CH3)2 N − CH2CH3
Write the IUPAC name of the following compound:
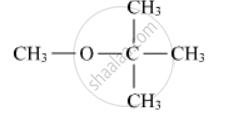
Write the structures of the products when Butan-2-ol reacts with SOCl2
Write the IUPAC name of the following :
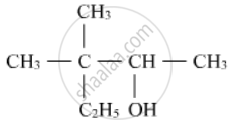
How do you convert the Ethanal to Propanone
In the dehydration of alcohols to alkenes by heating with concentrated sulphuric acid, the initiation step is:
(1) formation of carbonation
(2) formation of an ester
(3) protonation of the alcohol molecule
(4) elimination of water
What.will be the product fonned when chlorobenzene is heated with sodium metal in the presence of dry ether?
Propanoic acid to ethylamine.
Write structural formulae for 3-Methoxyhexane
Write structural formulae for Methyl vinyl ether.
Write structural formulae for 1-Ethylcyclohexanol.
Write structural formulae for Pentane-1,4-diol
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
Write IUPAC names of the following
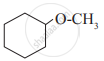
In a carbinol system of nomenclature tert.butyl alcohol is named as _______________
Isopropyl alcohol on oxidation forms:
C6H5OCH2CH3 is called:
3-methylphenol is called ____________.
One of the following is not a dihydroxy derivative of benzene.
The compound HOCH2 – CH2OH is __________.
Cresol has ____________.
Which of the following compounds is oxidised to prepare methyl ethyl ketone?
HBr reacts fastest with ____________.
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?
IUPAC name of m-cresol is ____________.
When ethyl alcohol reacts with acetic acid, the products formed are:
1-Propanol and 2-propanol can be best distinguished by:
The major product formed by the reaction:
\[\begin{array}{cc}
\ce{CH3CH-CH2Br ->[CH3O^-][CH3OH] is}\\
|\phantom{................}\\
\ce{CH3}\phantom{.............}
\end{array}\]
\[\ce{HC ≡ CH ->[HgSO4][H2SO4] ->[CH3MgBr][H2O] ->[PBr3]}\]
The product of acid catalysed hydration of 2-phenylpropene is:
The heating of phenyl methyl ether with HI produces:
\[\ce{Phenol ->[Zn, dust] 'X' ->[CH3Cl][Anhy. AlCl3] 'Y' ->[Alkaline][KMnO4] 'Z'}\]
The product ‘Z’ is:
Among the following sets of reactants which one produces anisole?
IUPAC name of the compound is:
\[\begin{array}{cc}
\ce{CH3-CH-OCH3}\\
|\phantom{....}\\
\ce{CH3}\phantom{..}
\end{array}\]
IUPAC name of m-cresol is ______.
Give IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH - CH2 - CH2 - CH - CH3}\phantom{.}\\
\phantom{.........}|\phantom{...................}|\phantom{...........}\\
\phantom{..}\ce{Cl}\phantom{.................}\ce{OH}\phantom{..}
\end{array}\]
Write the IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH2 - C = C - OH}\\
\phantom{........}|\phantom{....}|\phantom{}\\
\phantom{..............}\ce{CH3 CH2OH}
\end{array}\]
What happens when benzene diazonium chloride is heated with water?
Write steps to carry out the conversion of phenol to aspirin.
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (a) | 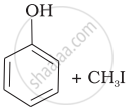 |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(c) | 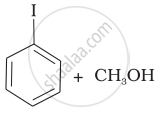 |
| (iv) | 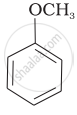 |
(d) | CH3—OH + CH3—I |
| (e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (f) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-I + CH3OH}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (g) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-OH + CH3I}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
Assertion: p-nitrophenol is more acidic than phenol.
Reason: Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
Assertion: IUPAC name of the compound
\[\begin{array}{cc}
\ce{CH3 - CH - O - CH2 - CH2 - CH3}\\
|\phantom{....................}\\
\ce{CH3}\phantom{.................}
\end{array}\] is 2-Ethoxy-2-methylethane.
Reason: In IUPAC nomenclature, ether is regarded as hydrocarbon derivative in which a hydrogen atom is replaced by —OR or —OAr group [where R = alkyl group and Ar = aryl group]
Assertion: Like bromination of benzene, bromination of phenol is also carried out in the presence of Lewis acid.
Reason: Lewis acid polarises the bromine molecule.
Assertion: Phenol forms 2, 4, 6 – tribromophenol on treatment with \[\ce{Br2}\] in carbon disulphide at 273 K.
Reason: Bromine polarises in carbon disulphide.
Assertion: Phenols give o- and p-nitrophenol on nitration with conc. \[\ce{HNO3}\] and \[\ce{H2SO4}\] mixture.
Reason: –OH group in phenol is o–, p– directing.
Explain why Lewis acid is not required in bromination of phenol?
Convert the following:
Ethyl alcohol into ethyl acetate
Write chemical reactions for the following conversion:
Acetic acid into ethyl alcohol
Identify A and B in the following:

Draw structure of the following compound.
2. 5-Diethylphenol
Draw structure of the following compound.
Prop-2-en-1-ol
Write structural formulae for:
Salicylic acid
The IUPAC name of 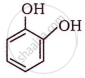 is ______.
is ______.
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{.....}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{.}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
