Advertisements
Advertisements
Question
What.will be the product fonned when chlorobenzene is heated with sodium metal in the presence of dry ether?
Solution
Chlorobenzene is heated with sodium metal in presence of dry ether gives diphenyl compound.

APPEARS IN
RELATED QUESTIONS
Write the IUPAC name of the given compound:
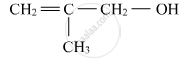
Write IUPAC name of the following compound:
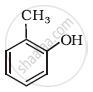
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
- Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
- Classify the isomers of alcohols in the above question as primary, secondary and tertiary alcohols.
Ethylidene dichloride when boiled with aqueous solution of NaOH yields _______.
(A) formaldehyde
(B) acetaldehyde
(C) acetone
(D) ethyl methyl ketone
Write IUPAC name of the following compound (CH3)2 N − CH2CH3
Propanoic acid to ethylamine.
Write structural formulae for 3-Methoxyhexane
Write structural formulae for Pentane-1,4-diol
Write structural formulae for Cyclohex-2-en-1-ol.
In a carbinol system of nomenclature tert.butyl alcohol is named as _______________
Give IUPAC names of the following compound:
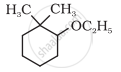
Butane-2-ol is ____________.
HBr reacts fastest with ____________.
IUPAC name of m-cresol is ____________.
\[\ce{Phenol ->[Zn, dust] 'X' ->[CH3Cl][Anhy. AlCl3] 'Y' ->[Alkaline][KMnO4] 'Z'}\]
The product ‘Z’ is:
Write steps to carry out the conversion of phenol to aspirin.
Assertion: p-nitrophenol is more acidic than phenol.
Reason: Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
Give the structures of Thiosulphuric acid and Peroxy monosulphuric acid.
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{.....}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{.}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
