Advertisements
Advertisements
Question
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{H2C = CH - CH - CH2 - CH2 - CH3}\\
|\phantom{..........}\\
\ce{OH}\phantom{........}
\end{array}\]
Solution
Hex-1-en-3-ol
APPEARS IN
RELATED QUESTIONS
Give IUPAC name of the following ether:
\[\begin{array}{cc}
\ce{C2H5OCH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
Write the structures of the products when Butan-2-ol reacts with SOCl2
In the dehydration of alcohols to alkenes by heating with concentrated sulphuric acid, the initiation step is:
(1) formation of carbonation
(2) formation of an ester
(3) protonation of the alcohol molecule
(4) elimination of water
Write IUPAC name of the following
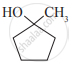
In a carbinol system of nomenclature tert.butyl alcohol is named as _______________
Isopropyl alcohol on oxidation forms:
Give IUPAC names of the following compound:
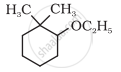
C6H5OCH2CH3 is called:
An example of a compound with functional group – O – is ____________.
Butane-2-ol is ____________.
The IUPAC name of the ether CH2 = CH–CH2OCH3 is:
The product of acid catalysed hydration of 2-phenylpropene is:
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(i) \[\ce{CrO3}\] in anhydrous medium.
(ii) \[\ce{KMnO4}\] in acidic medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573 K.
Write steps to carry out the conversion of phenol to aspirin.
Explain why Lewis acid is not required in bromination of phenol?
Identify A and B in the following:

Draw structure of the following compound.
2. 5-Diethylphenol
Draw structure of the following compound.
Prop-2-en-1-ol
