Advertisements
Advertisements
Question
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(i) \[\ce{CrO3}\] in anhydrous medium.
(ii) \[\ce{KMnO4}\] in acidic medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573 K.
Solution
(i) \[\ce{CrO3}\] in anhydrous medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573 K.
Explanation:
Strong oxidising agents such as acidified potassium permanganate are used for getting carboxylic acids from alcohols directly. \[\ce{CrO3}\] in anhydrous medium is used as the oxidising agent for the isolation of aldehydes.
\[\ce{RCH2OH ->[CrO3] RCHO}\]
A better reagent for the oxidation of primary alcohols to aldehydes in good yield is pyridinium chlorochromate (PCC), a complex for chromium trioxide with pyridine and HCl.
APPEARS IN
RELATED QUESTIONS
Natalite is a mixture of
(a) diethyl ether and methanol
(b) diethyl ether and ethanol
(c) dimethyl ether and methanol
(d) dimethyl ether and ethanol
What is the action of hot HI on it?
Write structural formulae for Methyl vinyl ether.
Write structural formulae for Pentane-1,4-diol
Write IUPAC names of the following
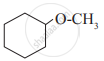
In a carbinol system of nomenclature tert.butyl alcohol is named as _______________
Which of the following gives a positive iodoform test?
Assertion: Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol.
Reason: Addition of water in acidic medium proceeds through the formation of primary carbocation.
Write complete reaction for the bromination of phenol in aqueous and non-aqueous medium.
How can phenol be converted to aspirin?
