Advertisements
Advertisements
Question
Assertion: Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol.
Reason: Addition of water in acidic medium proceeds through the formation of primary carbocation.
Options
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct statement but reason is wrong statement.
Assertion is wrong statement but reason is correct statement.
Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Solution
Assertion and reason both are wrong statements.
Explanation:
Addition of water to but-l-ene in acidic medium yields butan-2-ol.
Addition of water proceeds through formation of secondary carbocation.
APPEARS IN
RELATED QUESTIONS
Give IUPAC name of the following ether:
O2N – C6H4 – OCH3(p)
Give IUPAC name of the following ether:
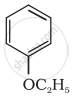
Write the IUPAC name of the following compound:
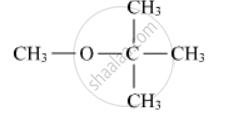
Write the structures of the products when Butan-2-ol reacts with CrO3
Write structural formulae for 1-Ethylcyclohexanol.
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
Give IUPAC names of the following compound:
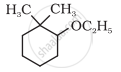
The IUPAC name of the ether CH2 = CH–CH2OCH3 is:
The heating of phenyl methyl ether with HI produces:
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{.....}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{.}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
