Advertisements
Advertisements
Question
Match the items of column I with items of column II.
| Column I | Column II | |
| (i) | Methanol | (a) Conversion of phenol to o-hydroxysalicylic acid |
| (ii) | Kolbe’s reaction | (b) Ethyl alcohol |
| (iii) | Williamson’s synthesis | (c) Conversion of phenol to salicylaldehyde |
| (iv) | Conversion of 2° alcohol to ketone | (d) Wood spirit |
| (v) | Reimer-Tiemann reaction | (e) Heated copper at 573 K |
| (vi) | Fermentation | (f) Reaction of alkyl halide with sodium alkoxide |
Solution
| Column I | Column II | |
| (i) | Methanol | (d) Wood spirit |
| (ii) | Kolbe’s reaction | (a) Conversion of phenol to o-hydroxysalicylic acid |
| (iii) | Williamson’s synthesis | (f) Reaction of alkyl halide with sodium alkoxide |
| (iv) | Conversion of 2° alcohol to ketone | (e) Heated copper at 573 K |
| (v) | Reimer-Tiemann reaction | (c) Conversion of phenol to salicylaldehyde |
| (vi) | Fermentation | (b) Ethyl alcohol |
Explanation:
(i) Methanol is also known as ‘wood spirit’ as it was produced by the destructive distillation of wood.
(ii) In Kolbe’s reaction, 2-hydroxybenzoic acid (salicylic acid) is prepared by the reaction of phenol with CO2 gas.
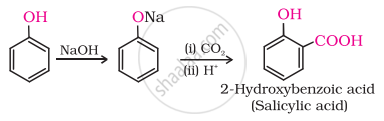
(iii) Williamson's synthesis is an important method for the preparation of either. In this method, an alkyl halide is allowed to react with sodium alkoxide.
\[\ce{R - X + R' - \overset{-}{\overset{\bullet\bullet}{\underset{\bullet\bullet}{O}}} \overset{+}{N}a -> R - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - R' + NaX}\]
(iv) When a 2° alcohol is allowed to pass overheated copper at 573 K, dehydrogenation takes place and an ketone is formed.
\[\begin{array}{cc}
\ce{R - CH - R' ->[Cu][573 K] R - C - R'}\\
|\phantom{...................}||\phantom{.}\\
\phantom{.}\ce{OH}\phantom{.................}\ce{O}\phantom{..}
\end{array}\]
(v) On treating phenol with chloroform in the presence of \[\ce{NaOH}\], an aldehydic group is introduced at ortho position of benzene ring

(vi) Ethanol is prepared by the fermentation of sugars.
\[\ce{C12H22O11 + H2O ->[Invertase] C6H12O6 + C6H12O6}\]
\[\ce{C6H12O6 ->[Zymase] 2C2H5OH + 2CO2}\]
APPEARS IN
RELATED QUESTIONS
Write resonance structures of aniline. What is the action of benzene diazonium chloride on ethanol?
If ethanol dissolves in water, then which of the following would be observed:
Wood spirit is known as acetone:
Suggest a reagent for conversion of ethanol to ethanoic acid.
Assertion: Ethanol is a weaker acid than phenol.
Reason: Sodium ethoxide may be prepared by the reaction of ethanol with aqueous \[\ce{NaOH}\].
Which reagent can convert acetic acid into ethanol?
Tonics in general contain
Liquor poisoning is due to
How methanol is obtained from methanal.
Give IUPAC names of the following compounds:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH - CH2-OH }\\
|\phantom{......}|\phantom{......}|\phantom{.......}\\
\ce{Cl}\phantom{....}\ce{CH3}\phantom{...}\ce{CH3}\phantom{.....}
\end{array}\]
