Advertisements
Advertisements
प्रश्न
Match the items of column I with items of column II.
| Column I | Column II | |
| (i) | Methanol | (a) Conversion of phenol to o-hydroxysalicylic acid |
| (ii) | Kolbe’s reaction | (b) Ethyl alcohol |
| (iii) | Williamson’s synthesis | (c) Conversion of phenol to salicylaldehyde |
| (iv) | Conversion of 2° alcohol to ketone | (d) Wood spirit |
| (v) | Reimer-Tiemann reaction | (e) Heated copper at 573 K |
| (vi) | Fermentation | (f) Reaction of alkyl halide with sodium alkoxide |
उत्तर
| Column I | Column II | |
| (i) | Methanol | (d) Wood spirit |
| (ii) | Kolbe’s reaction | (a) Conversion of phenol to o-hydroxysalicylic acid |
| (iii) | Williamson’s synthesis | (f) Reaction of alkyl halide with sodium alkoxide |
| (iv) | Conversion of 2° alcohol to ketone | (e) Heated copper at 573 K |
| (v) | Reimer-Tiemann reaction | (c) Conversion of phenol to salicylaldehyde |
| (vi) | Fermentation | (b) Ethyl alcohol |
Explanation:
(i) Methanol is also known as ‘wood spirit’ as it was produced by the destructive distillation of wood.
(ii) In Kolbe’s reaction, 2-hydroxybenzoic acid (salicylic acid) is prepared by the reaction of phenol with CO2 gas.
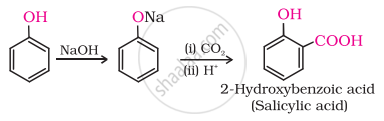
(iii) Williamson's synthesis is an important method for the preparation of either. In this method, an alkyl halide is allowed to react with sodium alkoxide.
\[\ce{R - X + R' - \overset{-}{\overset{\bullet\bullet}{\underset{\bullet\bullet}{O}}} \overset{+}{N}a -> R - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - R' + NaX}\]
(iv) When a 2° alcohol is allowed to pass overheated copper at 573 K, dehydrogenation takes place and an ketone is formed.
\[\begin{array}{cc}
\ce{R - CH - R' ->[Cu][573 K] R - C - R'}\\
|\phantom{...................}||\phantom{.}\\
\phantom{.}\ce{OH}\phantom{.................}\ce{O}\phantom{..}
\end{array}\]
(v) On treating phenol with chloroform in the presence of \[\ce{NaOH}\], an aldehydic group is introduced at ortho position of benzene ring

(vi) Ethanol is prepared by the fermentation of sugars.
\[\ce{C12H22O11 + H2O ->[Invertase] C6H12O6 + C6H12O6}\]
\[\ce{C6H12O6 ->[Zymase] 2C2H5OH + 2CO2}\]
APPEARS IN
संबंधित प्रश्न
Write the mechanism of the following reaction :
\[\ce{C2H5OH->[H2SO4][443K]CH2=CH2 + H2O}\]
Among the following the one which reacts most readily with ethanol is:
Methanol and ethanol are miscible in water due to ____________.
If ethanol dissolves in water, then which of the following would be observed:
Dipole moment of phenol is smaller than that of methanol. Why?
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Arrange water, ethanol and phenol in increasing order of acidity and give reason for your answer.
Alcoholic fermentation is brought about by the action of
Which of the following is known as wood spirit?
Write chemical reactions of following reagents on methoxyethane:
dilute H2SO4
