Advertisements
Advertisements
प्रश्न
Assertion: Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol.
Reason: Addition of water in acidic medium proceeds through the formation of primary carbocation.
विकल्प
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct statement but reason is wrong statement.
Assertion is wrong statement but reason is correct statement.
Both assertion and reason are correct statements but reason is not correct explanation of assertion.
उत्तर
Assertion and reason both are wrong statements.
Explanation:
Addition of water to but-l-ene in acidic medium yields butan-2-ol.
Addition of water proceeds through formation of secondary carbocation.
APPEARS IN
संबंधित प्रश्न
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{CH3 - C = C - CH2OH}\\
\phantom{}|\phantom{....}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{.}\ce{Br}\phantom{...}
\end{array}\]
Write IUPAC name of the following compound:
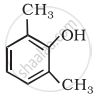
Write structural formulae for Cyclohex-2-en-1-ol.
Write IUPAC names of the following
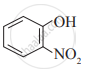
C6H5OCH2CH3 is called:
The product of acid catalysed hydration of 2-phenylpropene is:
What happens when benzene diazonium chloride is heated with water?
Arrange the following compounds in decreasing order of acidity.
\[\ce{H2O, ROH, HC ≡ CH}\]
Assertion: Phenol forms 2, 4, 6 – tribromophenol on treatment with \[\ce{Br2}\] in carbon disulphide at 273 K.
Reason: Bromine polarises in carbon disulphide.
Write complete reaction for the bromination of phenol in aqueous and non-aqueous medium.
