Advertisements
Advertisements
प्रश्न
Arrange the following compounds in decreasing order of acidity.
\[\ce{H2O, ROH, HC ≡ CH}\]
उत्तर
The decreasing order of the acidity of the given compounds:
\[\ce{H2O > ROH > HC ≡ CH}\]
\[\ce{HC ≡ CH}\] is less acidic because the carbon atoms here is sp hybridized, so the electron density is higher on the carbon atom.
APPEARS IN
संबंधित प्रश्न
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
|\phantom{......}|\phantom{..}\\
\ce{OH}\phantom{...}\ce{OH}\phantom{}
\end{array}\]
Give IUPAC name of the following ether:
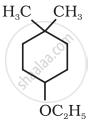
What is the action of hot HI on it?
Give reasons Fluoride ion has higher hydration enthalpy than chloride ion.
In the dehydration of alcohols to alkenes by heating with concentrated sulphuric acid, the initiation step is:
(1) formation of carbonation
(2) formation of an ester
(3) protonation of the alcohol molecule
(4) elimination of water
Write structural formulae for 3-Methoxyhexane
The IUPAC name of the ether CH2 = CH–CH2OCH3 is:
IUPAC name of the compound \[\begin{array}{cc}
\ce{CH3 - CH - OCH3}\\
\phantom{}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{..}
\end{array}\] is ______.
What happens when benzene diazonium chloride is heated with water?
Draw structure of the following compound.
2-Methoxypropane
