Advertisements
Advertisements
Question
Arrange the following compounds in decreasing order of acidity.
\[\ce{H2O, ROH, HC ≡ CH}\]
Solution
The decreasing order of the acidity of the given compounds:
\[\ce{H2O > ROH > HC ≡ CH}\]
\[\ce{HC ≡ CH}\] is less acidic because the carbon atoms here is sp hybridized, so the electron density is higher on the carbon atom.
APPEARS IN
RELATED QUESTIONS
Write the IUPAC name of the given compound:
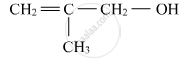
Write IUPAC name of the following compound:
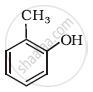
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH2 - O - CH - CH2 - CH3}\\
\phantom{...}|\\
\phantom{.....}\ce{CH3}
\end{array}\]
How is phenol converted into the following?
benzoquinone
The compound HOCH2 – CH2OH is __________.
IUPAC name of the compound is:
\[\begin{array}{cc}
\ce{CH3-CH-OCH3}\\
|\phantom{....}\\
\ce{CH3}\phantom{..}
\end{array}\]
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(i) \[\ce{CrO3}\] in anhydrous medium.
(ii) \[\ce{KMnO4}\] in acidic medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573 K.
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (a) | 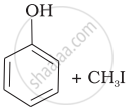 |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(c) | 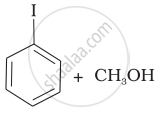 |
| (iv) | 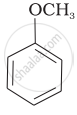 |
(d) | CH3—OH + CH3—I |
| (e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (f) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-I + CH3OH}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (g) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-OH + CH3I}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
Draw structure of the following compound.
2. 5-Diethylphenol
The IUPAC name of 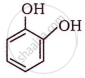 is ______.
is ______.
