Advertisements
Advertisements
Question
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH2 - O - CH - CH2 - CH3}\\
\phantom{...}|\\
\phantom{.....}\ce{CH3}
\end{array}\]
Solution
2-Ethoxybutane
APPEARS IN
RELATED QUESTIONS
Explain metamerism with suitable examples of ethers
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{........................}\ce{CH2OH}\\
\phantom{..................}|\\
\ce{CH3 - CH - CH2 - CH - CH - CH3}\\
\phantom{}|\phantom{.............}|\phantom{........}\\
\phantom{..}\ce{CH3}\phantom{..........}\ce{OH}\phantom{........}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
|\phantom{......}|\phantom{..}\\
\ce{OH}\phantom{...}\ce{OH}\phantom{}
\end{array}\]
Write IUPAC name of the following compound:
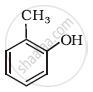
How is phenol converted into the following?
picric acid
How do you convert the Ethanal to Propanone
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
Write IUPAC names of the following
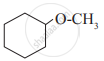
Glycerol is ____________.
C6H5OCH2CH3 is called:
Ethyl alcohol is industrially prepared from ethylene by:
IUPAC name of m-cresol is ____________.
Which of the following gives a positive iodoform test?
Write steps to carry out the conversion of phenol to aspirin.
Explain why p-nitrophenol is more acidic than phenol.
Assertion: Like bromination of benzene, bromination of phenol is also carried out in the presence of Lewis acid.
Reason: Lewis acid polarises the bromine molecule.
Assertion: Phenol forms 2, 4, 6 – tribromophenol on treatment with \[\ce{Br2}\] in carbon disulphide at 273 K.
Reason: Bromine polarises in carbon disulphide.
Explain why Lewis acid is not required in bromination of phenol?
Draw structure of the following compound.
2. 5-Diethylphenol
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
