Advertisements
Advertisements
Question
Explain why Lewis acid is not required in bromination of phenol?
Solution
Usual halogenation is carried out in the presence of Lewis acid, \[\ce{FeBr3}\] which polarises the halogen molecule. In case of phenol, the polarisation of bromine occurs even in the absence of Lewis acid. This is because of highly activating effect of –OH group on the benzene ring. The reaction follows:
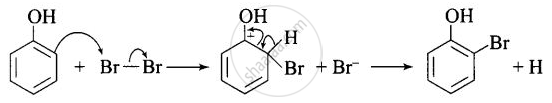
Note: In aqueous solution, phenol ionizes to give phenoxide ion. Due to the presence of the negative charge, the oxygen atom of the phenoxide ion donates electrons to the benzene ring to a large extent. As a result, the ring gets highly activated leading to the formation of trisubstituted product. On the other hand, in the non-polar solvents, the ionization of phenol does not occurs to a large extent. As a result, the -OH group donates electrons to the benzene ring only to a small extent. Consequently, the ring is activated slightly and, therefore, only monosubstitution occurs.
APPEARS IN
RELATED QUESTIONS
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{.}}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
- Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
- Classify the isomers of alcohols in the above question as primary, secondary and tertiary alcohols.
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
Which of the following compounds is NOT prepared by the action of alcoholic NI3 on alkyl halide?
(a) CH3NH2
(b) CH3- CH2- NH2
(c) CH3 - CH2 - CH2 - NH2
(d) (CH3)3 C- NH2
Write structural formulae for 1-Ethylcyclohexanol.
IUPAC name of m-cresol is ____________.
The major product formed by the reaction:
\[\begin{array}{cc}
\ce{CH3CH-CH2Br ->[CH3O^-][CH3OH] is}\\
|\phantom{................}\\
\ce{CH3}\phantom{.............}
\end{array}\]
IUPAC name of the compound \[\begin{array}{cc}
\ce{CH3 - CH - OCH3}\\
\phantom{}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{..}
\end{array}\] is ______.
Write structural formulae for:
p-Nitrophenol
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
