Advertisements
Advertisements
Question
Write structural formulae for 1-Ethylcyclohexanol.
Solution
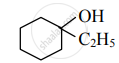
1-Ethylcyclohexanol
APPEARS IN
RELATED QUESTIONS
Explain metamerism with suitable examples of ethers
Write the IUPAC name of the given compound:
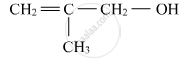
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
Name the following compound according to IUPAC system.
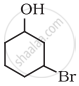
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{CH3 - C = C - CH2OH}\\
\phantom{}|\phantom{....}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{.}\ce{Br}\phantom{...}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{.}}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{HO - CH2 - CH - CH2 - OH}\\
|\phantom{..}\\
\ce{OH}
\end{array}\]
Write IUPAC name of the following compound:
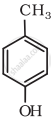
Write IUPAC name of the following compound:
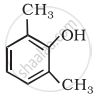
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - O - CH2 - CH - CH3}\\
\phantom{..........}|\\
\phantom{............}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH2 - O - CH - CH2 - CH3}\\
\phantom{...}|\\
\phantom{.....}\ce{CH3}
\end{array}\]
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
- Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
- Classify the isomers of alcohols in the above question as primary, secondary and tertiary alcohols.
Give IUPAC name of the following ether:
\[\begin{array}{cc}
\ce{C2H5OCH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
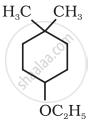
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
Give IUPAC name of the following ether:
Which of the following compounds is NOT prepared by the action of alcoholic NI3 on alkyl halide?
(a) CH3NH2
(b) CH3- CH2- NH2
(c) CH3 - CH2 - CH2 - NH2
(d) (CH3)3 C- NH2
Write the structure and IUPAC name of 'methyl-n-propyl ether'.
What is the action of hot HI on it?
How is phenol converted into the following?
picric acid
Write IUPAC name of the following compound (CH3)2 N − CH2CH3
Give reasons Fluoride ion has higher hydration enthalpy than chloride ion.
Write the IUPAC name of the following compound:
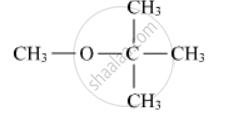
Write the structures of the products when Butan-2-ol reacts with CrO3
Write the structures of the products when Butan-2-ol reacts with SOCl2
Write the IUPAC name of the following :
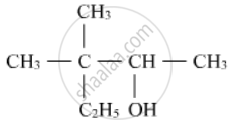
How do you convert the Ethanal to Propanone
What.will be the product fonned when chlorobenzene is heated with sodium metal in the presence of dry ether?
Propanoic acid to ethylamine.
Write structural formulae for Pentane-1,4-diol
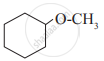
Write IUPAC names of the following
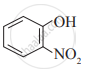
Write IUPAC names of the following
In a carbinol system of nomenclature tert.butyl alcohol is named as _______________
Glycerol is ____________.
Isopropyl alcohol on oxidation forms:
Give IUPAC names of the following compound:
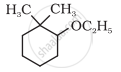
C6H5OCH2CH3 is called:
3-methylphenol is called ____________.
One of the following is not a dihydroxy derivative of benzene.
An example of a compound with functional group – O – is ____________.
Butane-2-ol is ____________.
Cresol has ____________.
Which of the following compounds is oxidised to prepare methyl ethyl ketone?
HBr reacts fastest with ____________.
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?
IUPAC name of m-cresol is ____________.
When ethyl alcohol reacts with acetic acid, the products formed are:
Which of the following is most acidic?
The IUPAC name of the ether CH2 = CH–CH2OCH3 is:
The major product formed by the reaction:
\[\begin{array}{cc}
\ce{CH3CH-CH2Br ->[CH3O^-][CH3OH] is}\\
|\phantom{................}\\
\ce{CH3}\phantom{.............}
\end{array}\]
\[\ce{HC ≡ CH ->[HgSO4][H2SO4] ->[CH3MgBr][H2O] ->[PBr3]}\]
Which of the following gives a positive iodoform test?
\[\ce{Phenol ->[Zn, dust] 'X' ->[CH3Cl][Anhy. AlCl3] 'Y' ->[Alkaline][KMnO4] 'Z'}\]
The product ‘Z’ is:
IUPAC name of the compound is:
\[\begin{array}{cc}
\ce{CH3-CH-OCH3}\\
|\phantom{....}\\
\ce{CH3}\phantom{..}
\end{array}\]
IUPAC name of m-cresol is ______.
IUPAC name of the compound \[\begin{array}{cc}
\ce{CH3 - CH - OCH3}\\
\phantom{}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{..}
\end{array}\] is ______.
Give IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH - CH2 - CH2 - CH - CH3}\phantom{.}\\
\phantom{.........}|\phantom{...................}|\phantom{...........}\\
\phantom{..}\ce{Cl}\phantom{.................}\ce{OH}\phantom{..}
\end{array}\]
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(i) \[\ce{CrO3}\] in anhydrous medium.
(ii) \[\ce{KMnO4}\] in acidic medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573 K.
Write the IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH2 - C = C - OH}\\
\phantom{........}|\phantom{....}|\phantom{}\\
\phantom{..............}\ce{CH3 CH2OH}
\end{array}\]
What happens when benzene diazonium chloride is heated with water?
Explain why p-nitrophenol is more acidic than phenol.
Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
| Column I | Column II | |
| (i) | 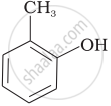 |
(a) Hydroquinone |
| (ii) | 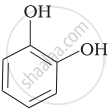 |
(b) Phenetole |
| (iii) | 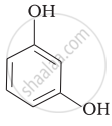 |
(c) Catechol |
| (iv) | 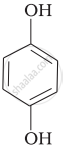 |
(d) o-Cresol |
| (v) | 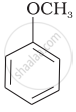 |
(e) guinone |
| (vi) | 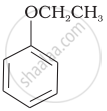 |
(f) Resorcinol |
| (g) Anisole |
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (a) | 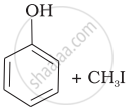 |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(c) | 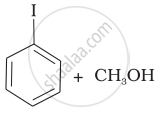 |
| (iv) | 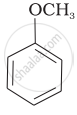 |
(d) | CH3—OH + CH3—I |
| (e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (f) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-I + CH3OH}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (g) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-OH + CH3I}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
Assertion: Addition reaction of water to but-1-ene in acidic medium yields butan-1-ol.
Reason: Addition of water in acidic medium proceeds through the formation of primary carbocation.
Assertion: p-nitrophenol is more acidic than phenol.
Reason: Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
Assertion: IUPAC name of the compound
\[\begin{array}{cc}
\ce{CH3 - CH - O - CH2 - CH2 - CH3}\\
|\phantom{....................}\\
\ce{CH3}\phantom{.................}
\end{array}\] is 2-Ethoxy-2-methylethane.
Reason: In IUPAC nomenclature, ether is regarded as hydrocarbon derivative in which a hydrogen atom is replaced by —OR or —OAr group [where R = alkyl group and Ar = aryl group]
How can phenol be converted to aspirin?
Write the IUPAC name of the following compound.
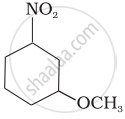
Convert the following:
Ethyl alcohol into ethyl acetate
Write chemical reactions for the following conversion:
Acetic acid into ethyl alcohol
Identify A and B in the following:

How are the following conversions carried out?
Methyl magnesium bromide→2-Methylpropan-2-ol.
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Draw structure of the following compound.
2. 5-Diethylphenol
Draw structure of the following compound.
Prop-2-en-1-ol
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{.....}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{.}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write IUPAC names of the following compounds:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3\phantom{...}OH\phantom{...}CH3}\\
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
