Advertisements
Advertisements
Question
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
Solution
3-Chloromethyl-2-isopropylpentan-1-ol
APPEARS IN
RELATED QUESTIONS
Name the following compound according to IUPAC system.
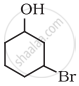
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
|\phantom{......}|\phantom{..}\\
\ce{OH}\phantom{...}\ce{OH}\phantom{}
\end{array}\]
Write IUPAC name of the following compound:
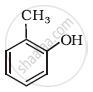
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
3-Methylbutane-2-ol on heating with HI gives ______
Write IUPAC name of the following compound (CH3)2 N − CH2CH3
Give reasons Fluoride ion has higher hydration enthalpy than chloride ion.
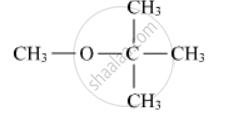
Write the IUPAC name of the following compound:
What.will be the product fonned when chlorobenzene is heated with sodium metal in the presence of dry ether?
Write structural formulae for Pentane-1,4-diol
An example of a compound with functional group – O – is ____________.
Ethylene reacts with Baeyer’s reagent to give ______.
Explain why p-nitrophenol is more acidic than phenol.
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (a) | 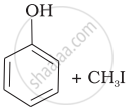 |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(c) | 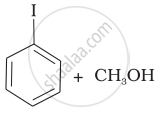 |
| (iv) | 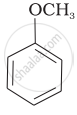 |
(d) | CH3—OH + CH3—I |
| (e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (f) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-I + CH3OH}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (g) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-OH + CH3I}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
Write complete reaction for the bromination of phenol in aqueous and non-aqueous medium.
Write chemical reactions for the following conversion:
Acetic acid into ethyl alcohol
How are the following conversions carried out?
Methyl magnesium bromide→2-Methylpropan-2-ol.
Draw structure of the following compound.
Prop-2-en-1-ol
Write structural formulae for:
Salicylic acid
