Advertisements
Advertisements
Question
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{........................}\ce{CH2OH}\\
\phantom{..................}|\\
\ce{CH3 - CH - CH2 - CH - CH - CH3}\\
\phantom{}|\phantom{.............}|\phantom{........}\\
\phantom{..}\ce{CH3}\phantom{..........}\ce{OH}\phantom{........}
\end{array}\]
Solution
2, 5-Dimethylhexane-1, 3-diol
APPEARS IN
RELATED QUESTIONS
Explain metamerism with suitable examples of ethers
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\ce{H2C = CH - CH - CH2 - CH2 - CH3}\\
|\phantom{..........}\\
\ce{OH}\phantom{........}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
|\phantom{......}|\phantom{..}\\
\ce{OH}\phantom{...}\ce{OH}\phantom{}
\end{array}\]
Natalite is a mixture of
(a) diethyl ether and methanol
(b) diethyl ether and ethanol
(c) dimethyl ether and methanol
(d) dimethyl ether and ethanol
Write the structure and IUPAC name of 'methyl-n-propyl ether'.
How is phenol converted into the following?
picric acid
In the dehydration of alcohols to alkenes by heating with concentrated sulphuric acid, the initiation step is:
(1) formation of carbonation
(2) formation of an ester
(3) protonation of the alcohol molecule
(4) elimination of water
Give IUPAC names of the following compound:
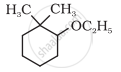
Give IUPAC names of the following compound:
\[\begin{array}{cc}
\phantom{..}\ce{H}\phantom{...}\ce{CH3}\phantom{.}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{}\\
\ce{H - C - C - C - H}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{}\\
\phantom{.}\ce{H}\phantom{...}\ce{OH}\phantom{.}\ce{H}\phantom{.}\\
\end{array}\]
One of the following is not a dihydroxy derivative of benzene.
IUPAC name of m-cresol is ____________.
\[\ce{HC ≡ CH ->[HgSO4][H2SO4] ->[CH3MgBr][H2O] ->[PBr3]}\]
Which of the following gives a positive iodoform test?
Write the IUPAC name of the compound given below.
\[\begin{array}{cc}
\phantom{}\ce{CH3 - CH2 - C = C - OH}\\
\phantom{........}|\phantom{....}|\phantom{}\\
\phantom{..............}\ce{CH3 CH2OH}
\end{array}\]
Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
| Column I | Column II | |
| (i) | 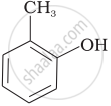 |
(a) Hydroquinone |
| (ii) | 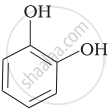 |
(b) Phenetole |
| (iii) | 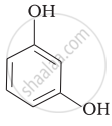 |
(c) Catechol |
| (iv) | 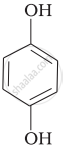 |
(d) o-Cresol |
| (v) | 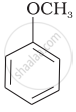 |
(e) guinone |
| (vi) | 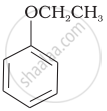 |
(f) Resorcinol |
| (g) Anisole |
Assertion: p-nitrophenol is more acidic than phenol.
Reason: Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
How are the following conversions carried out?
Methyl magnesium bromide→2-Methylpropan-2-ol.
Draw structure of the following compound.
Prop-2-en-1-ol
Write structural formulae for:
p-Nitrophenol
