Advertisements
Advertisements
Question
Explain why p-nitrophenol is more acidic than phenol.
Solution
The electron-withdrawing group (−NO2), withdraws electrons and disperses the negative charge. Therefore, the −NO2 group stabilizes the phenoxide ion. Hence p-nitrophenol is more acidic than phenol.
APPEARS IN
RELATED QUESTIONS
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
3-Methylbutane-2-ol on heating with HI gives ______
How is phenol converted into the following?
benzoquinone
Write the IUPAC name of the following :
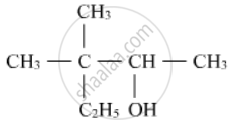
In the dehydration of alcohols to alkenes by heating with concentrated sulphuric acid, the initiation step is:
(1) formation of carbonation
(2) formation of an ester
(3) protonation of the alcohol molecule
(4) elimination of water
Write structural formulae for Methyl vinyl ether.
IUPAC name of m-cresol is ____________.
Among the following sets of reactants which one produces anisole?
Write steps to carry out the conversion of phenol to aspirin.
How can phenol be converted to aspirin?
