Advertisements
Advertisements
Question
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
Solution
2-Chloro-1-methoxyethane
APPEARS IN
RELATED QUESTIONS
What is metamerism?
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{........................}\ce{CH2OH}\\
\phantom{..................}|\\
\ce{CH3 - CH - CH2 - CH - CH - CH3}\\
\phantom{}|\phantom{.............}|\phantom{........}\\
\phantom{..}\ce{CH3}\phantom{..........}\ce{OH}\phantom{........}
\end{array}\]
Write IUPAC name of the following compound:
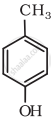
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
Natalite is a mixture of
(a) diethyl ether and methanol
(b) diethyl ether and ethanol
(c) dimethyl ether and methanol
(d) dimethyl ether and ethanol
Write the IUPAC name of the following :
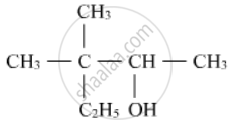
Write structural formulae for 1-Ethylcyclohexanol.
Write structural formulae for Pentane-1,4-diol
Cresol has ____________.
Ethylene reacts with Baeyer’s reagent to give ______.
IUPAC name of the compound \[\begin{array}{cc}
\ce{CH3 - CH - OCH3}\\
\phantom{}|\phantom{....}\\
\phantom{}\ce{CH3}\phantom{..}
\end{array}\] is ______.
Assertion: Phenols give o- and p-nitrophenol on nitration with conc. \[\ce{HNO3}\] and \[\ce{H2SO4}\] mixture.
Reason: –OH group in phenol is o–, p– directing.
Explain why Lewis acid is not required in bromination of phenol?
Draw structure of the following compound.
2-Methoxypropane
Write structural formulae for:
Salicylic acid
The IUPAC name of 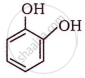 is ______.
is ______.
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{.....}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{.}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{...............}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{..}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
