Advertisements
Advertisements
प्रश्न
Give IUPAC name of the following ether:
CH3OCH2CH2Cl
उत्तर
2-Chloro-1-methoxyethane
APPEARS IN
संबंधित प्रश्न
Write the IUPAC name of the given compound:
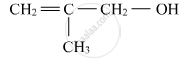
Write structures of the compounds whose IUPAC names are as follows:
3-Chloromethylpentan-1-ol.
Give IUPAC name of the following ether:
CH3CH2CH2OCH3
Natalite is a mixture of
(a) diethyl ether and methanol
(b) diethyl ether and ethanol
(c) dimethyl ether and methanol
(d) dimethyl ether and ethanol
Which of the following compounds is NOT prepared by the action of alcoholic NI3 on alkyl halide?
(a) CH3NH2
(b) CH3- CH2- NH2
(c) CH3 - CH2 - CH2 - NH2
(d) (CH3)3 C- NH2
3-Methylbutane-2-ol on heating with HI gives ______
Give reasons Fluoride ion has higher hydration enthalpy than chloride ion.
Write the structures of the products when Butan-2-ol reacts with CrO3
Propanoic acid to ethylamine.
Write structural formulae for Methyl vinyl ether.
Give IUPAC names of the following compound:
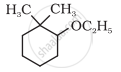
The compound HOCH2 – CH2OH is __________.
The product of acid catalysed hydration of 2-phenylpropene is:
Arrange the following compounds in decreasing order of acidity.
\[\ce{H2O, ROH, HC ≡ CH}\]
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
| Column I | Column II | ||
| (i) | CH3—O—CH3 | (a) | 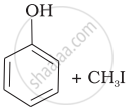 |
| (ii) | \[\begin{array}{cc} \ce{CH3}\phantom{..................}\\ \backslash\phantom{.............}\\ \ce{CH-O-CH3}\\ /\phantom{..............}\\ \ce{CH3}\phantom{..................} \end{array}\] |
(b) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-I + CH3OH}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | \[\begin{array}{cc} \ce{CH3}\phantom{.}\\ |\phantom{....}\\ \ce{H3C-C-O-CH3}\\ |\phantom{....}\\ \ce{CH3}\phantom{..} \end{array}\] |
(c) | 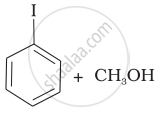 |
| (iv) | 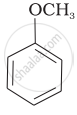 |
(d) | CH3—OH + CH3—I |
| (e) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-OH + CH3I}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (f) | \[\begin{array}{cc} \ce{CH3}\phantom{.....................}\\ \backslash\phantom{.................}\\ \ce{CH-I + CH3OH}\\ /\phantom{.................}\\ \ce{CH3}\phantom{.....................} \end{array}\] |
||
| (g) | \[\begin{array}{cc} \ce{CH3}\phantom{....}\\ |\phantom{.......}\\ \ce{CH3-C-OH + CH3I}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
Assertion: Phenol forms 2, 4, 6 – tribromophenol on treatment with \[\ce{Br2}\] in carbon disulphide at 273 K.
Reason: Bromine polarises in carbon disulphide.
Convert the following:
Ethyl alcohol into ethyl acetate
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{................}\ce{CH3}\\
\phantom{.............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{......}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{...}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
