Advertisements
Advertisements
प्रश्न
Give reasons Fluoride ion has higher hydration enthalpy than chloride ion.
उत्तर
Fluoride ion is smaller in size than the chloride ion. Hence, when the two are dissolved in water, the energy of hydration released in the case of fluoride ion will be more than chloride ion due to stronger interactions (ion-dipole) between the ion and the water molecules in the former. Fluorine being more electronegative than chlorine will form stronger H-bonds with hydrogen present in the water molecule.
`triangleH_("hyd")F^(-) > triangle H_("hyd") Cl^(-)`
संबंधित प्रश्न
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{............}\ce{CH2OH}\\
\phantom{......}|\\
\ce{CH3 - CH2 - CH - CH - CH - CH3}\\
\phantom{......}|\phantom{............}|\phantom{.}\\
\phantom{........}\ce{CH2Cl}\phantom{......}\ce{CH3}\phantom{}
\end{array}\]
Name the following compound according to IUPAC system.
\[\begin{array}{cc}
\phantom{........................}\ce{CH2OH}\\
\phantom{..................}|\\
\ce{CH3 - CH - CH2 - CH - CH - CH3}\\
\phantom{}|\phantom{.............}|\phantom{........}\\
\phantom{..}\ce{CH3}\phantom{..........}\ce{OH}\phantom{........}
\end{array}\]
Give IUPAC name of the following ether:
\[\begin{array}{cc}
\ce{C2H5OCH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{.......}\ce{CH3}
\end{array}\]
3-Methylbutane-2-ol on heating with HI gives ______
Write IUPAC name of the following compound (CH3)2 N − CH2CH3
Write structural formulae for Pentane-1,4-diol
The compound HOCH2 – CH2OH is __________.
IUPAC name of the compound is:
\[\begin{array}{cc}
\ce{CH3-CH-OCH3}\\
|\phantom{....}\\
\ce{CH3}\phantom{..}
\end{array}\]
Write the IUPAC name of the following compound.
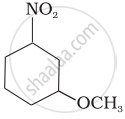
Write the IUPAC name of the following compound:
\[\begin{array}{cc}
\phantom{..............}\ce{CH3}\\
\phantom{............}|\\
\ce{CH3 - CH - CH - C - CH3}\\
|\phantom{.....}|\phantom{......}|\\
\phantom{...}\ce{CH3}\phantom{.}\ce{OH}\phantom{...}\ce{CH3}
\end{array}\]
