Advertisements
Advertisements
Question
Which of the following is most acidic?
Options
Benzyl alcohol
Cyclohexanol
Phenol
m-Chlorophenol
Solution
m-Chlorophenol
Explanation:
m-chlorophenol is most acidic. Alpha carbon of benzyl alcohol and cyclohexanol is sp3 hybridized. In m-chlorophenol, it is sp2 hybridized. In m-chlorophenol, electron-withdrawing group \[\ce{-Cl}\] is present at meta position.
APPEARS IN
RELATED QUESTIONS
Ethylidene dichloride when boiled with aqueous solution of NaOH yields _______.
(A) formaldehyde
(B) acetaldehyde
(C) acetone
(D) ethyl methyl ketone
Write the structure and IUPAC name of 'methyl-n-propyl ether'.
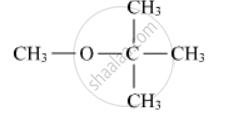
Write the IUPAC name of the following compound:
Write structural formulae for Pentane-1,4-diol
Isopropyl alcohol on oxidation forms:
Ethylene reacts with Baeyer’s reagent to give ______.
Which of the following gives a positive iodoform test?
Which of the following compounds will react with sodium hydroxide solution in water?
Explain why Lewis acid is not required in bromination of phenol?
Write structural formulae for:
p-Nitrophenol
