Advertisements
Online Mock Tests
Chapters
2: Solutions
3: Ionic Equilibria
4: Chemical Thermodynamics
5: Electrochemistry
6: Chemical Kinetics
7: Elements of Groups 16, 17 and 18
8: Transition and Inner transition Elements
9: Coordination Compounds
10: Halogen Derivatives
▶ 11: Alcohols, Phenols and Ethers
12: Aldehydes, Ketones and Carboxylic acids
13: Amines
14: Biomolecules
15: Introduction to Polymer Chemistry
16: Green Chemistry and Nanochemistry
![Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 11 - Alcohols, Phenols and Ethers Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 11 - Alcohols, Phenols and Ethers - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:74400b801d4c44ef8e058ff9d9dfe964.jpg)
Advertisements
Solutions for Chapter 11: Alcohols, Phenols and Ethers
Below listed, you can find solutions for Chapter 11 of Maharashtra State Board Balbharati for Chemistry [English] 12 Standard HSC.
Balbharati solutions for Chemistry [English] 12 Standard HSC 11 Alcohols, Phenols and Ethers Exercises [Pages 252 - 253]
Choose the most correct option.
Choose the correct option.
Which of the following represents the increasing order of boiling points of (1), (2), and (3)?
(1) CH3 - CH2 - CH2 - CH2 - OH
(2) (CH3)2CHOCH3
(3) (CH3)3COH
(1) < (2) < (3)
(2) < (1) < (3)
(3) < (2) < (1)
(2) < (3) < (1)
Which is the best reagent for carrying out the following conversion?

\[\ce{LiAlH4}\]
Conc. \[\ce{H2SO4, H2O}\]
\[\ce{H2/Pd}\]
\[\ce{B2H6, H2O2-NaOH}\]
Choose the correct option.
Which of the following substrate will give ionic organic products on reaction?
CH3 – CH2 – OH + Na
CH3 – CH2 – OH + SOCl2
CH3 – CH2 – OH + PCl5
CH3 – CH2 – OH + H2SO4
Choose the correct option.
Which is the most resistant alcohol towards oxidation reaction among the following?
CH3 - CH2 - OH
(CH3)2CH - OH
(CH3)3C - OH
\[\begin{array}{cc}\ce{C2H5 CH - OH }\\|\phantom{}\\
\ce{CH3\phantom{}}\end{array}\]
Resorcinol on distillation with zinc dust gives _________.
Cyclohexane
Benzene
Toluene
Benzene-1, 3-diol
Anisole on heating with concerntrated HI gives _________.
Iodobenzene
Phenol + Methanol
Phenol + Iodomethane
Iodobenzene + methanol
Choose the correct option.
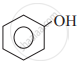
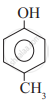
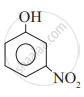
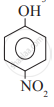
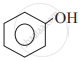
Which of the following is the least acidic compound?
Choose the correct option.
The compound incapable of hydrogen bonding with water is _________.
CH3-CH2-O-CH3
CH3-CH2-CH2-CH3
CH3-CH2-CH2-OH
Choose the correct option.
Ethers are kept in air tight brown bottles because ______________.
Ethers absorb moisture
Ethers evaporate readily
Ethers oxidise to explosive peroxide
Ethers are inert
Choose the correct option.
Ethers react with cold and concentrated H2SO4 to form ________.
oxonium salt
alkene
alkoxides
alcohols
Answer in one sentence/ word.
Hydroboration-oxidation of propene gives _________.
Answer in one sentence/ word.
Write the IUPAC name of alcohol having molecular formula C4H10O which is resistant towards oxidation.
Answer in one sentence/ word.
Write the structure of optically active alcohol having molecular formula C4H10O
Answer in one sentence/ word.
Write the name of the electrophile used in Kolbe’s Reaction.
Answer in brief.
Explain why phenol is more acidic than ethyl alcohol.
Answer in brief.
Explain why p-nitrophenol is a stronger acid than phenol.
Write two points of difference between the properties of phenol and ethyl alcohol.
Give the reagents and conditions necessary to prepare phenol from Chlorobenzene.
Answer in brief.
Give the reagents and conditions necessary to prepare phenol from Benzene sulfonic acid.
Answer in brief.
Give the equations of the reactions for the preparation of phenol from isopropyl benezene.
Answer in brief.
Give a simple chemical test to distinguish between ethanol and ethyl bromide.
An ether (A), C5H12O, when heated with excess of hot HI produce two alkyl halides which on hydrolysis form compound (B) and (C), oxidation of (B) gave and acid (D), whereas oxidation of (C) gave a ketone (E). Deduce the structural formula of (A), (B), (C), (D), and (E).
Write structural formulae for 3-Methoxyhexane
Write structural formulae for Methyl vinyl ether.
Write structural formulae for 1-Ethylcyclohexanol.
Write structural formulae for Pentane-1,4-diol
Write structural formulae for Cyclohex-2-en-1-ol.
Write IUPAC name of the following
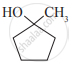
Write IUPAC name of the following
\[\begin{array}{cc}\ce{CH3-CH-CH-CH2-OH}\\|\phantom{.....}|\phantom{.......}\\\ce{OH}\phantom{..}\ce{CH3}\phantom{.....}\end{array}\]
Write IUPAC names of the following
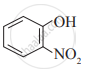
Write IUPAC names of the following
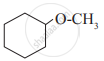
Solutions for 11: Alcohols, Phenols and Ethers
![Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 11 - Alcohols, Phenols and Ethers Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 11 - Alcohols, Phenols and Ethers - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:74400b801d4c44ef8e058ff9d9dfe964.jpg)
Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 11 - Alcohols, Phenols and Ethers
Shaalaa.com has the Maharashtra State Board Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Balbharati solutions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board 11 (Alcohols, Phenols and Ethers) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Balbharati textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] 12 Standard HSC chapter 11 Alcohols, Phenols and Ethers are Alcohols, Phenols and Ethers, Classification of Alcohols, Phenols and Ethers, Nomenclature, Alcohols and Phenols, Ethers, Uses of Alcohols, Phenols and Ethers.
Using Balbharati Chemistry [English] 12 Standard HSC solutions Alcohols, Phenols and Ethers exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Balbharati Solutions are essential questions that can be asked in the final exam. Maximum Maharashtra State Board Chemistry [English] 12 Standard HSC students prefer Balbharati Textbook Solutions to score more in exams.
Get the free view of Chapter 11, Alcohols, Phenols and Ethers Chemistry [English] 12 Standard HSC additional questions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board, and you can use Shaalaa.com to keep it handy for your exam preparation.