Advertisements
Online Mock Tests
Chapters
2: Solutions
3: Ionic Equilibria
4: Chemical Thermodynamics
5: Electrochemistry
6: Chemical Kinetics
7: Elements of Groups 16, 17 and 18
8: Transition and Inner transition Elements
9: Coordination Compounds
10: Halogen Derivatives
11: Alcohols, Phenols and Ethers
12: Aldehydes, Ketones and Carboxylic acids
▶ 13: Amines
14: Biomolecules
15: Introduction to Polymer Chemistry
16: Green Chemistry and Nanochemistry
![Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 13 - Amines Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 13 - Amines - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:74400b801d4c44ef8e058ff9d9dfe964.jpg)
Advertisements
Solutions for Chapter 13: Amines
Below listed, you can find solutions for Chapter 13 of Maharashtra State Board Balbharati for Chemistry [English] 12 Standard HSC.
Balbharati solutions for Chemistry [English] 12 Standard HSC 13 Amines Exercises [Pages 296 - 297]
Choose the most correct option.
The hybridisation of nitrogen in primary amine is ____________.
sp
sp2
sp3
sp3d
Isobutylamine is an example of ______.
2° amine
3° amine
1° amine
quaternary ammonium salt
Choose the most correct option.
Which one of the following compounds has the highest boiling point?
n-Butylamine
sec-Butylamine
isobutylamine
tert-Butylamine
Choose the most correct option.
Which of the following has the highest basic strength?
Trimethylamine
Methylamine
Ammonia
Dimethylamine
Choose the most correct option.
Which type of amine does produce N2 when treated with HNO2?
Primary amine
Secondary amine
Tertiary amine
Both primary and secondary amines
Choose the most correct option.
Carbylamine test is given by ____________.
Primary amine
Secondary amine
Tertiary amine
Both secondary and tertiary amines
Which one of the following compounds does not react with acetyl chloride?
CH3 - CH2 - NH2
(CH3 - CH2)2NH
(CH3 - CH2)3N
C6H5 - NH2
Choose the most correct option.
Which of the following compounds will dissolve in aqueous NaOH after undergoing reaction with Hinsberg reagent?
Ethylamine
Triethylamine
Trimethylamine
Diethylamine
Choose the most correct option.
Identify ‘B’ in the following reactions
\[\ce{CH3 - C ≡ N ->[Na/C2H5OH] A ->[NaNO2/dilHCl]B}\]
CH3 - CH2 - NH2
CH3 - CH2 - NO2
CH3 - CH2N2+Cl-
CH3 - CH2 - OH
Choose the most correct option.
Which of the following compounds contains azo linkage?
Hydrazine
p-Hydroxyazobenzene
N-Nitrosodiethylamine
Ethylenediamine
Answer in one sentence.
Write the reaction of p-toluenesulphonyl chloride with diethylamine.
Answer in one sentence.
How many moles of methylbromide is required to convert ethanamine to N, N-dimethyl ethanamine?
Answer in one sentence.
Which amide does produce ethanamine by Hofmann bromamide degradation reaction?
Answer in one sentence.
Write the order of the basicity of aliphatic alkylamine in the gaseous phase.
Answer in one sentence.
Why are primary aliphatic amines stronger bases than ammonia?
Answer in one sentence.
Predict the product of the following reaction.
\[\ce{Nitrobenzene ->[Sn/conc.HCl]?}\]
Answer in one sentence.
Write the IUPAC name of benzylamine.
Answer in one sentence.
Arrange the following amines in increasing order of boiling points.
n-propylamine, ethylmethyl amine, trimethylamine.
Answer in one sentence.
Write the balanced chemical equations for the action of dil H2SO4 on diethylamine.
Answer in one sentence.
Arrange the following amines in the increasing order of their pKb values.
Aniline, Cyclohexylamine, 4-Nitroaniline
Answer the following
Identify A and B in the following reactions.
\[\ce{C6H5CH2Br->[alco.][KCN]A ->[Na/ethanol]B.}\]
Explain the basic nature of amines with a suitable example.
Answer the following
What is diazotisation?
Answer the following
Write diazotisation reaction of aniline?
Answer the following
Write a reaction to convert acetic acid into methylamine.
Write a short note on coupling reactions.
Answer the following
Explain Gabriel phthalimide synthesis.
Answer the following
Explain carbylamine reaction with suitable examples.
Answer the following
Write a reaction to convert methanamine into ethanamine.
Answer the following
Write a reaction to convert Aniline into p-bromoaniline.
Answer the following
Complete the following reaction :
`"C"_6"H"_5"N"_2^+"Cl"^-` + C2H5OH →
Answer the following
Complete the following reaction :
\[\ce{C6H5NH2 + Br2_{(aq)} -> ?}\]
Answer the following
Explain the ammonolysis of alkyl halides.
Answer the following
Write a reaction to convert ethylamine into methylamine.
Answer the following.
Write the IUPAC name of the following amine :
\[\begin{array}{cc}
\ce{CH3 - CH2 - N - CH2 - CH2 - CH3}\\
|\phantom{........}\\
\ce{CH3\phantom{......}}
\end{array}\]
Answer the following.
Write the IUPAC name of the following amine :
\[\begin{array}{cc}\\
\ce{CH3}\phantom{...........}\\
|\phantom{..............}\\
\ce{CH3 - C - CH2 - CH2 - NH2}\\
|\phantom{..............}\\
\ce{CH3\phantom{...........}}
\end{array}\]
Answer the following.
Write the IUPAC name of the following amine :
\[\begin{array}{cc}\\
\ce{CH3 - CH - NH - CH2 - CH3}\\
|\phantom{...............}\\
\ce{CH3\phantom{............}}
\end{array}\]
What are amines?
How are amines classified?
Answer the following.
Write the IUPAC name of the following amine.
H2N-(CH2)6 - NH2
Answer the following.
Write the IUPAC name of the following amine.

Answer the following.
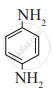
Write the IUPAC name of the following amine.
Write reactions to prepare ethanamine from Acetonitrile.
Answer the following.
Write a reaction to prepare ethanamine from Nitroethane
Answer the following.
Write a reaction to prepare ethanamine from Propionamide.
Answer the following.
What is the action of acetic anhydride on ethylamine?
Answer the following.
What is the action of acetic anhydride on diethylamine?
Answer the following.
What is the action of acetic anhydride on triethylamine?
Distinguish between ethylamine, diethylamine, and triethylamine by using Hinsberg’s reagent?
Answer the following.
Write a reaction to bring about the following conversions: Aniline into p-nitroaniline
Answer the following.
Write a reaction to bring about the following conversions: Aniline into sulphanilic acid?
Solutions for 13: Amines
![Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 13 - Amines Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 13 - Amines - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:74400b801d4c44ef8e058ff9d9dfe964.jpg)
Balbharati solutions for Chemistry [English] 12 Standard HSC chapter 13 - Amines
Shaalaa.com has the Maharashtra State Board Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Balbharati solutions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board 13 (Amines) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Balbharati textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] 12 Standard HSC chapter 13 Amines are Classification of Amines, Nomenclature of Amines, Preparation of Amines, Physical Properties of Amines, Basicity of Amines, Chemical Properties of Amines, Reactions of Arene Diazonium Salts, Reaction with Arenesulfonyl Chloride, Electrophilic Aromatic Substitution in Aromatic Amines.
Using Balbharati Chemistry [English] 12 Standard HSC solutions Amines exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Balbharati Solutions are essential questions that can be asked in the final exam. Maximum Maharashtra State Board Chemistry [English] 12 Standard HSC students prefer Balbharati Textbook Solutions to score more in exams.
Get the free view of Chapter 13, Amines Chemistry [English] 12 Standard HSC additional questions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board, and you can use Shaalaa.com to keep it handy for your exam preparation.
